CCS has emerged as a new and crucial concept underscored in the latest
revision of Annex 1 in 2022, reflecting its significance with 52 mentions throughout the
guidance.
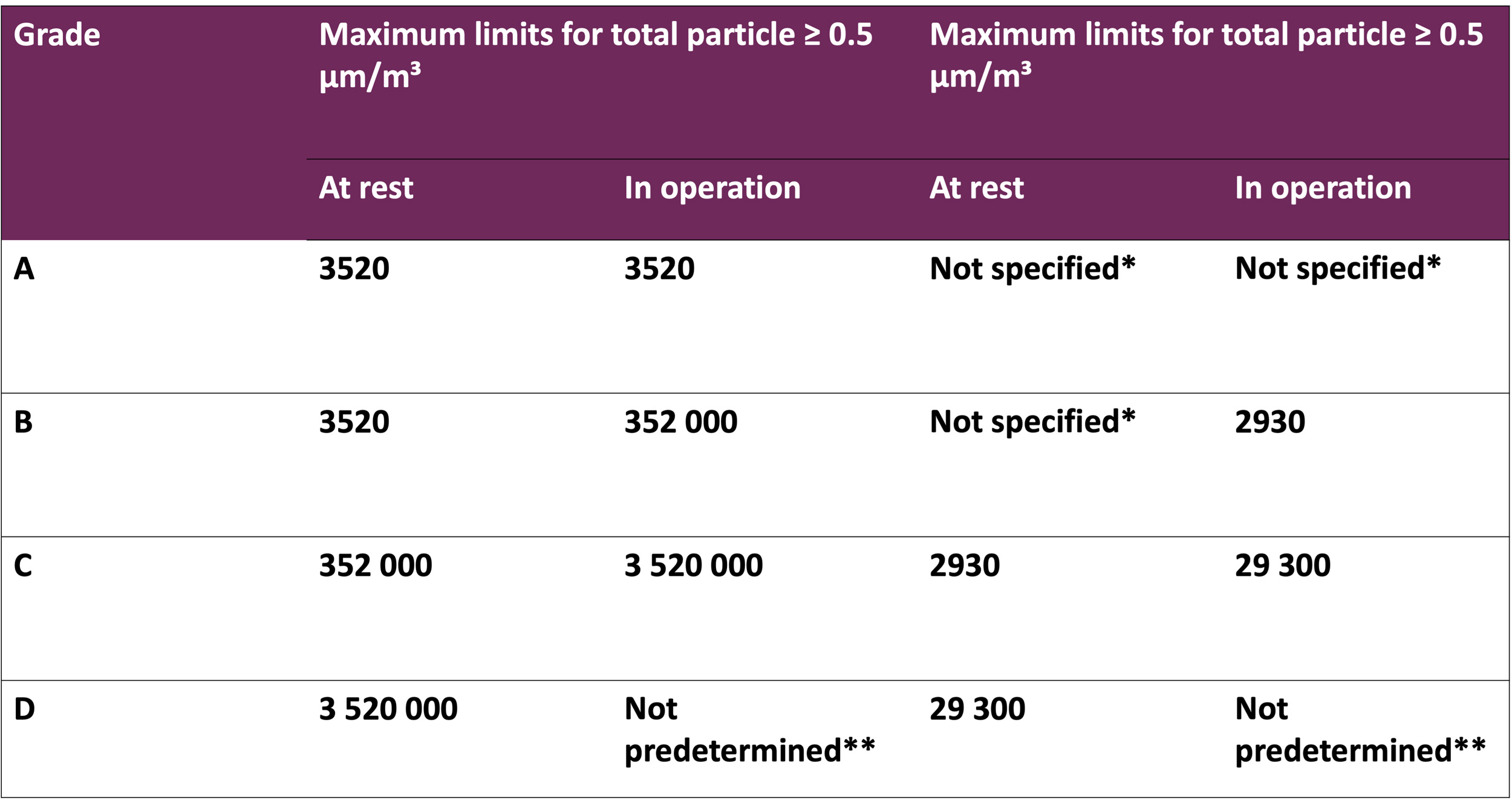
It is a concept that expands beyond the manufacture of sterile products to encompass
design of premises, cleanroom classification, qualification, validation, monitoring and personnel
gowning. Moreover, it serves as a supportive framework for the production of non-sterile
products.
A notable statement on page 5 of Annex 1 highlights the pivotal role of senior management
in ensuring effective oversight of control measures throughout the facility and product lifecycle.
Within this context, the mention of CCS occurs nine times within Section 2 and a
total of 52 times throughout the entire document, reflecting its heightened importance. This increased
emphasis on CCS and QRM underscores the growing recognition of their pivotal role in ensuring product
quality, safety, and compliance with regulatory standards.
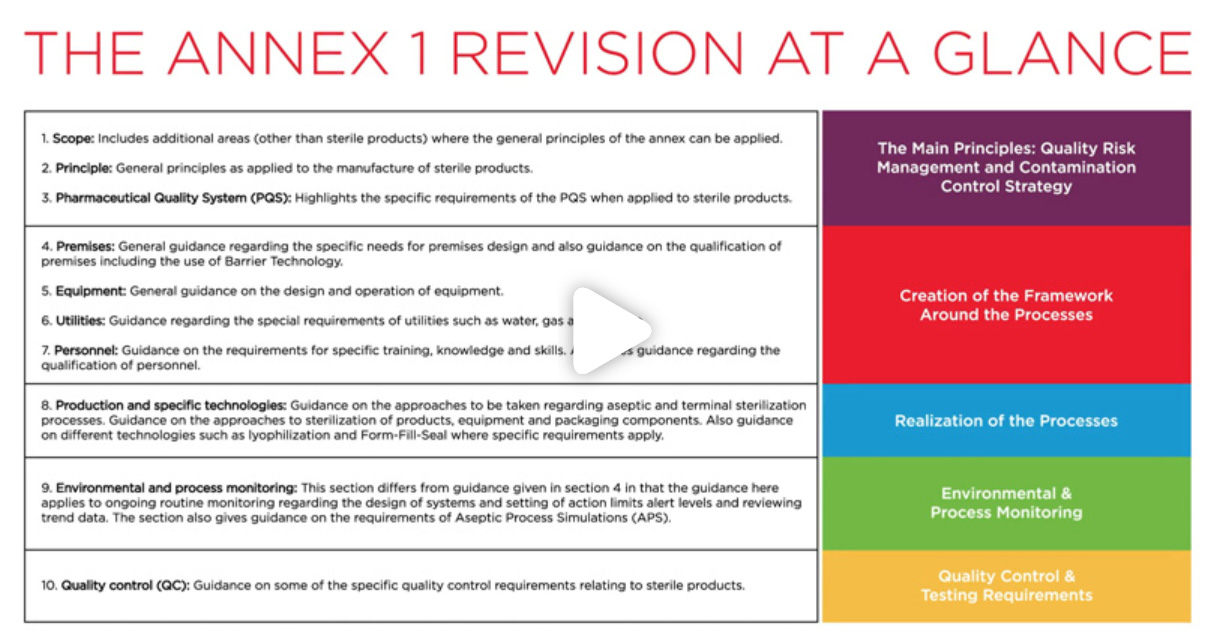
The evolution of these guidelines underscores a significant shift from the
previous absence of developed principles in 2008. The updated guidelines now dedicate Section 2 to
articulate detailed expectations and areas of consideration. It emphasizes the imperative for managing
processes, equipment, facilities, and manufacturing activities in accordance with QRM principles.