Products & ReviewClinical Diagnostics
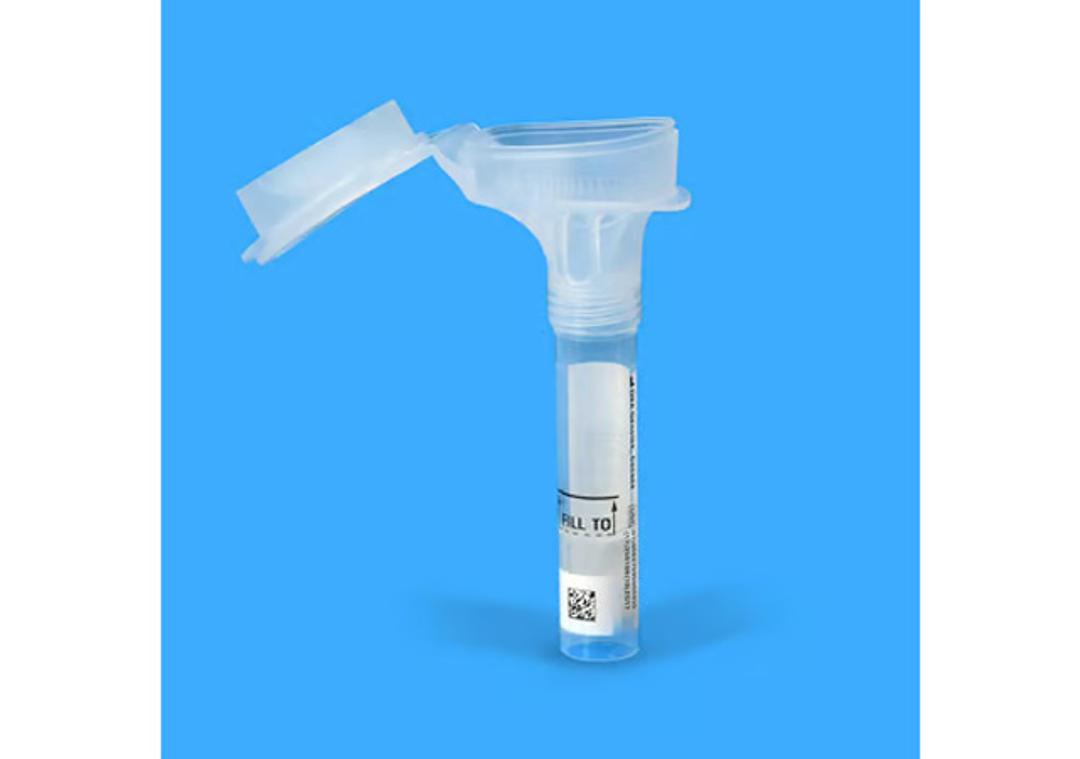
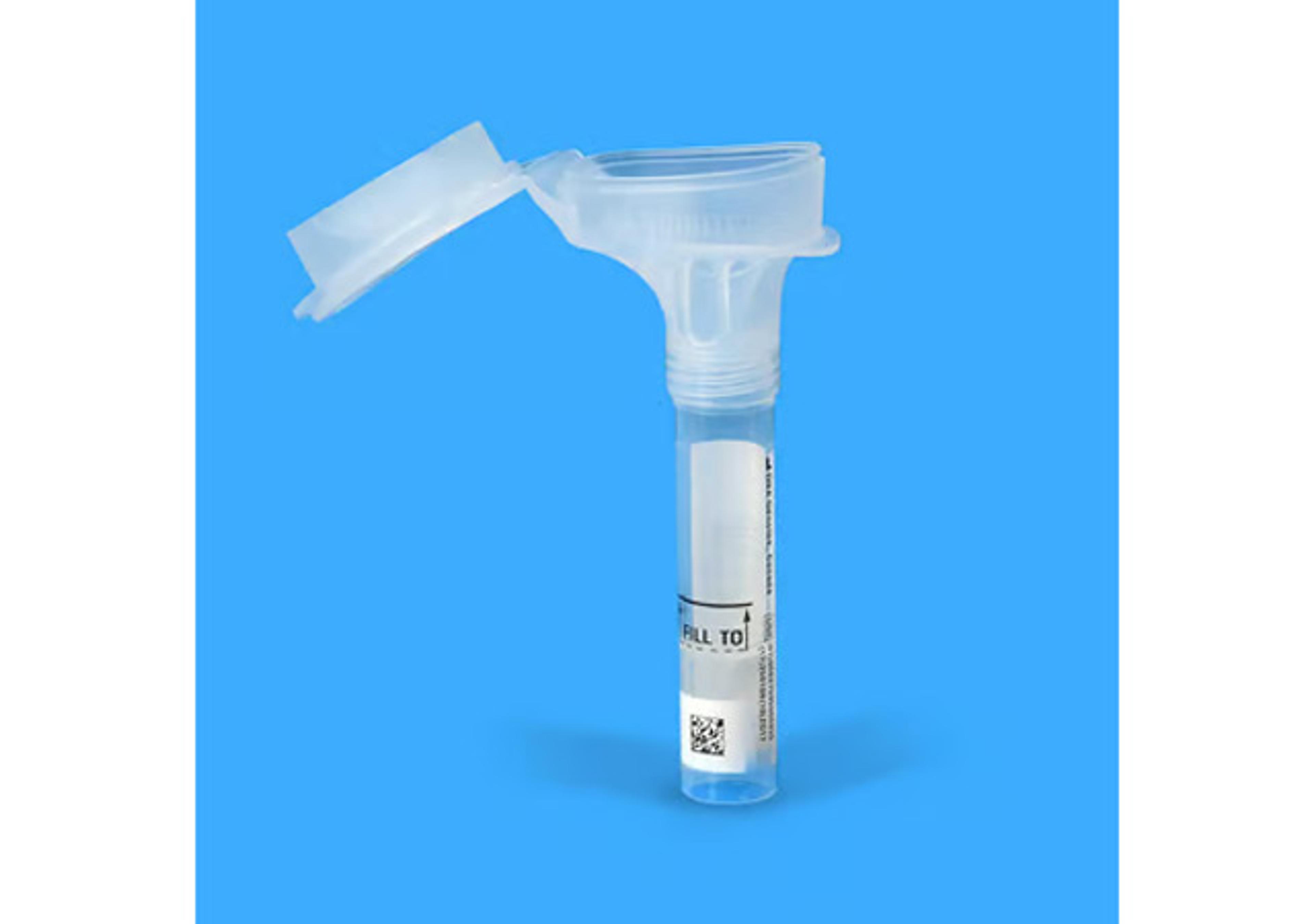
Oragene™•Dx saliva collection device
Collect high quality DNA samples for molecular diagnostics with an FDA cleared device.
- U.S. FDA 510(k) general clearance for prescription (Rx) and direct-to-consumer (DTC) uses
- Leveraging the 510(k) clearance of Oragene™•Dx device may reduce the time and complexity of an FDA submission
- All-in-one system for the collection, stabilization and transportation of DNA from saliva
- Painless, minimally-invasive and easy-to-use DNA sample device for self collection
Device / Assay manufacturers must validate the use of this device for their specific indications for use.