7 essential resources in pharmaceutical quality assurance
Explore the latest technologies, free downloads, and expert insights that can help your laboratory achieve compliance successfully
13 Dec 2021

As part of our new special feature, we’ve pulled together a selection of the top resources and technologies to help you meet quality and compliance requirements.
Read on to explore the importance of quality control of raw materials used in IVD manufacturing, focused on the production of high-quality antibodies used in COVID-19 diagnostic testing. Plus, find out more about the technology designed to help you determine product purity, access guides that can help you meet compliance regulations with ease, and more.

IMMUNOASSAYS: A close look at in vitro diagnostic manufacturing
In this article, Minna Mattila, Medix Biochemica, shares her expert insights on the importance of quality control in the development of IVD immunoassays for COVID-19, including the key analytical technologies to ensure quality control.
PURITY: Comparing SDS-PAGE with Maurice CE-SDS
In this application note, discover how to improve your protein purity characterization with Maurice by ProteinSimple, offering significantly higher peak profile resolution with more accurate quantitation of impurities than other traditional SDS-PAGE slab-gel methods.

RAW MATERIALS: Raman analysis of pharmaceutical ingredients
In this application note, Ocean Insight illustrates the capabilities of Raman spectroscopy in the analysis of pharmaceutical active ingredients, helping to maintain quality and consistency.

QUALITY MANAGEMENT: Weighing according to the European Pharmacopoeia (Ph. Eur.)
In this white paper, see a description of the weighing requirements according to the European Pharmacopoeia General Chapter 2.1.7 ‘Balances for analytical purposes' and ensure compliance by Jan 1, 2022.

PHARMACEUTICAL PRODUCTS: Keeping particulate contamination under control
Discover how a 2-methods-in-1 solution combining optical microscopy and laser-induced breakdown spectroscopy (LIBS) can be utilized for identification of particulate contaminants in the pharmaceutical industry.
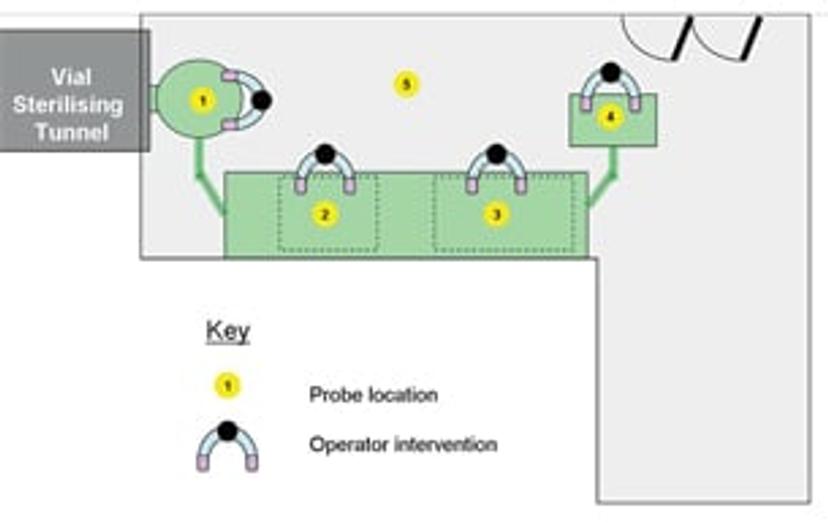
ENVIRONMENTAL MONITORING: GMP cleanrooms classification
Designing and validating your Cleanroom Routine Environmental Monitoring and Classification SOPs is time consuming and complex. Learn about the differences between GMP Cleanroom Classification and Routine Environmental Monitoring, and solutions to help.

DATA INTEGRITY: Real results from bridging gaps in data management
In this case study, discover how one major biopharmaceutical company improved process development and regulatory submission efficiencies.
More top pharmaceutical assurance resources:
Ensuring the quality of your medications: Watch this presentation, from the SelectScience® Virtual Biopharma Summit 2021, where Dr. Jared R. Auclair, Director of the Biopharmaceutical Analysis Laboratory (BATL), Northeastern University, discusses the importance of quality control in manufacturing. Watch video»
Validated pharmaceutical testing: Looking for the best way to integrate UV-Vis technology into a pharmaceutical QA/QC or research environment? This webinar explains the tools and technology available for instrument validation and qualification, data integrity, and more. Watch on demand»
Cleanrooms: In this application note, find out how to meet Good Manufacturing Practice (GMP) guidelines when establishing and maintaining a cleanroom. Download info»
Contract manufacturing: The commercialization pathway for IVD devices is complicated, but a knowledgeable contract manufacturing (CM) partner can save significant time and money. Discover the key considerations to help guide your decision when it comes to choosing a CM partner.Download info»
Lab weighing: In this application eBook, we explore the importance of weighing compliance in the regulated lab and provide you with the tools to achieve greater lab efficiency.Download eBook»
Your recommendations:
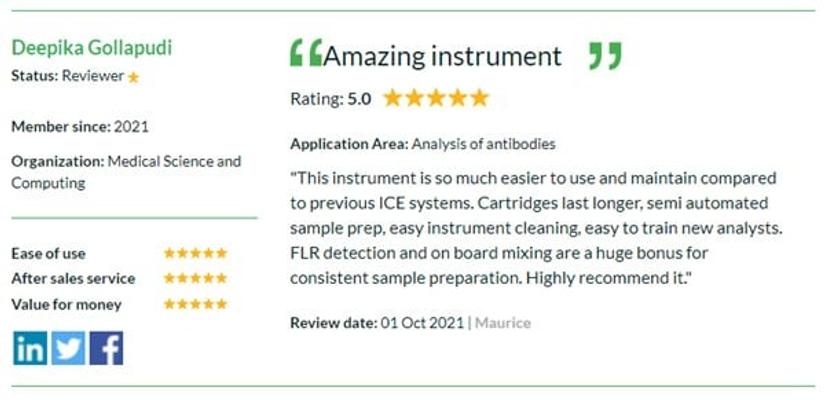
Take a look at what other researchers all over the world are saying about the latest equipment and technologies helping scientists achieve compliance. Here, Deepika Gollapudi, Medical Science and Computing, shares her opinion on the Maurice by ProteinSimple.
Find more news and resources in our Pharmaceutical Quality Assurance Special Feature >>