A translationally-relevant alternative to non-human primates
The Jackson Laboratory explores the efficacy of humanized mice as an alternative to non-human primates in drug development studies
29 May 2022
The Food and Drug Administration (FDA) has published recommendations to drug developers and researchers urging them to consider alternatives to non-human primates (NHPs) in general toxicology studies due to COVID-related shortages. This FDA document is the latest example of a trend started years ago to reduce NHP use in preclinical research.
NHPs are often considered the gold standard to assess the toxicity of immunostimulatory antibodies and related products since they share the highest level of gene homology with humans compared to the other preclinical animal models. The replacement of NHPs with in vitro assays risks missing cell non-autonomous toxic interactions that might happen only in vivo, such as the mechanism underlying the cytokine release syndrome (CRS). On the other hand, most in vivo preclinical models (such as rodents, dogs, and rabbits) lack the human-specific drug target and have very little translational value.
The hu-PBMC-NSG model is a translationally relevant experimental platform to evaluate immunotoxicity
Designed to reproduce a functional human immune system on a smaller scale, JAX hu-PBMC-NSG mice are NSG™ immunodeficient mice engrafted with pre-characterized human peripheral blood mononuclear cells (PBMCs). The engrafted mice evolve from simply a “rodent model” to a translationally-relevant in vivo platform. The translational value of hu-PBMC-NSG mice becomes obvious in CRS evaluation studies. For example, unlike most preclinical assays currently available to drug developers, the hu-PBMC-NSG platform allows for the detection of the toxicity of a human-specific activator molecule such as the CD28 superagonist TGN1412 (Fig 1).
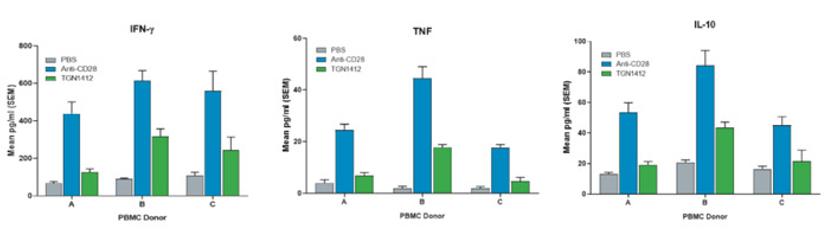
Utilizing the hu-PBMC-NSG model as a platform to evaluate the immunotoxicity of monoclonal and bispecific antibodies
In contrast to NHPs, researchers can engraft hu-PBMC-NSG mice with tumors to assess the effect of target engagement on the immunotoxicity elicited by the compound. This feature is crucial when testing the CRS potential of bispecific antibodies such as CD3 engagers, which require the concomitant engagement of both arms to express their activity and potential toxicity (Fig. 2). In fact, one could argue that hu-PBMC-NSG mice are more relevant than NHPs for the toxicological evaluation of immuno-oncology drugs since researchers can evaluate the immunotoxicity in the presence of the target tumor.
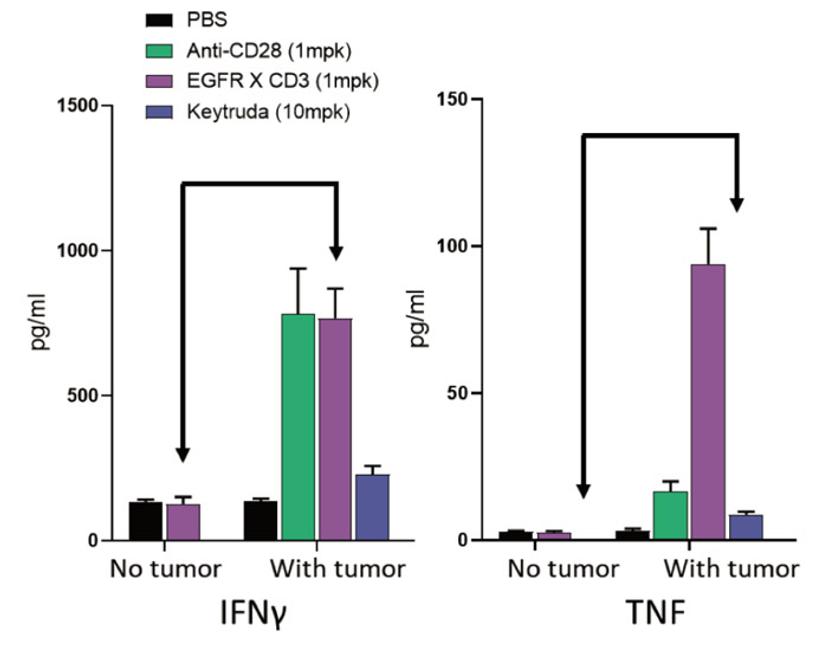
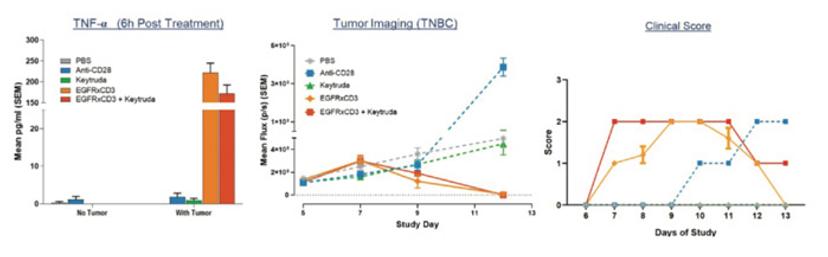
Using the hu-PBMC-NSG platform to evaluate the immunotoxicity of drug combinations
An additional use of hu-PBMC-NSG mice is predicting the CRS potential of drug combinations. While cost, ethical, and practical considerations limit the usage of NHPs in testing multiple drug combinations, hu-PBMC-NSG mice provide translational results that inform researchers about the potential toxicity and efficacy of specific drug combinations. Figure 3 illustrates an example where a CD3 engager targeting EGFR was combined with Keytruda. The results show that the combination does not increase the treatment toxicity, but does not yield any efficacy advantage either.
The hu-PBMC-NSG model is a translationally-relevant tool to test the immunotoxicity of CAR T therapies
The response of hu-PBMC-NSGs to bispecific antibodies is also mirrored with CAR T cell therapies, which is particularly important since many patients see a severe toxic reaction in the form of CRS. Traditional in vitro assays cannot mimic the systemic immune response that causes downstream organ and neurological toxicities seen in patients, a crucial feature for determining the true efficacy and safety of the therapeutic.
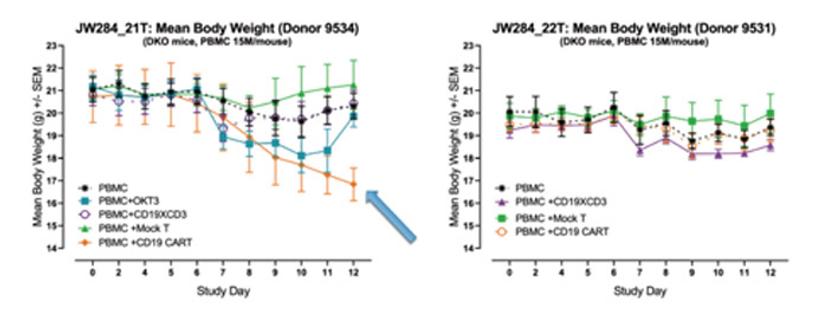
In an assay that is completed in less than 16 days, the humanized mouse platform is focused on individual patient responses, where CAR T efficacy and CRS can be measured together using individually characterized PBMC donors, simulating patient responses seen in a clinical setting (Figure 4).
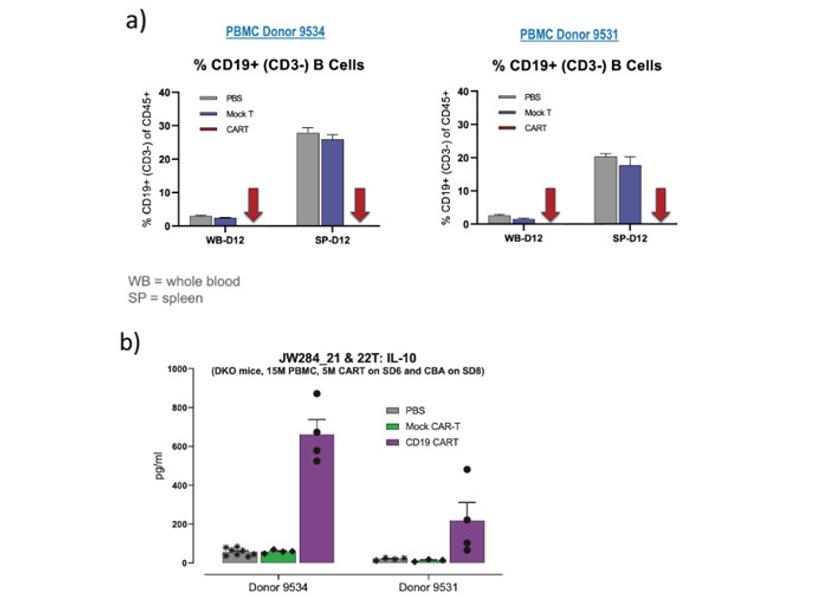
A significant advantage of in vivo models over in vitro approaches is the possibility of mimicking complex, multi-organ responses that result in complex phenotypes such as clinical manifestations and body weight loss. For example, when examining the body weight data for the same study as the previous two figures, we see significant differences in body weight consistent with the cytokine levels. This data supports the holistic value in assessing CAR T in an in vivo assay with a humanized platform to examine the overall impact of the cytokine response on the health of the mice, which cannot be achieved using in vitro or a non-humanized platform.
The use of a humanized system that combines a fully functional human immune system with human targets offers a more predictive data set that better mirrors the diversity and variance seen within a unique human population. For more information of the use of humanized mouse platforms for antibody pharmacokinetic, efficacy, and toxicity studies, please visit the resources below or contact a JAX expert.

