Access the cellular social network with EV characterization
Perform quantitative analyses of the biological nanoparticles and demonstrate properties for cellular communication and disease progression or inhibition
30 Aug 2023
Image © drmicrobe @123rf.com
Extracellular vesicles (EVs) have great potential for influencing the diagnosis of diseases such as cancer and may provide solutions for therapeutics. These unique cell-secreted particles, ranging from a few nanometers to a few microns in diameter, offer an exclusive insight into the cellular world. Their involvement in removing material from the cell and their delivery to recipient cells has them carrying proteins, lipids, and RNAs, a goldmine of potential biomarkers. In fact, in 2022, 50% of the EV-related clinical trials documented were in biomarker applications.
Simultaneously, their ability to travel efficiently through barriers such as the blood-brain barrier makes them a promising candidate for therapeutics.
The NanoAnalyzer, developed by NanoFCM, uses a laser system for individual particle measurement, performing accurate sizing, simultaneous counting, and determination of fluorescent markers. This nano-flowcytometry (nFCM) approach allows for comprehensive identification of target molecules, but its design allows for dynamic sub-population examination.
Home in on specific EV sub-populations
In a study by Cancers 2022, the NanoAnalyzer was used to explain the correlation between the percentage of EVs, derived from ascites of ovarian cancer patients, with platelet markers and the PDW/PLT surrogate indicator of intravascular platelet activation (Figure 1).1
Two fluorescent labelling strategies were used to first distinguish EVs from a complex biofluid isolation and then further identify those EVs presenting platelet-associated markers GPIIb/IIIa and PF4. In ovarian cancer, platelets contribute to the formation and expansion of the early metastatic niche and their activation is associated with poor prognosis.
The combined analyses of all >45nm particles with specific characterization of sub-populations of interest allowed for deconvolution of data, predicting a correlation between % of EVs with platelet markers and the PDW/PLT. This could allow for biofluid extractions to be used in assessing the extent of platelet activation in ascites of ovarian cancer patients.
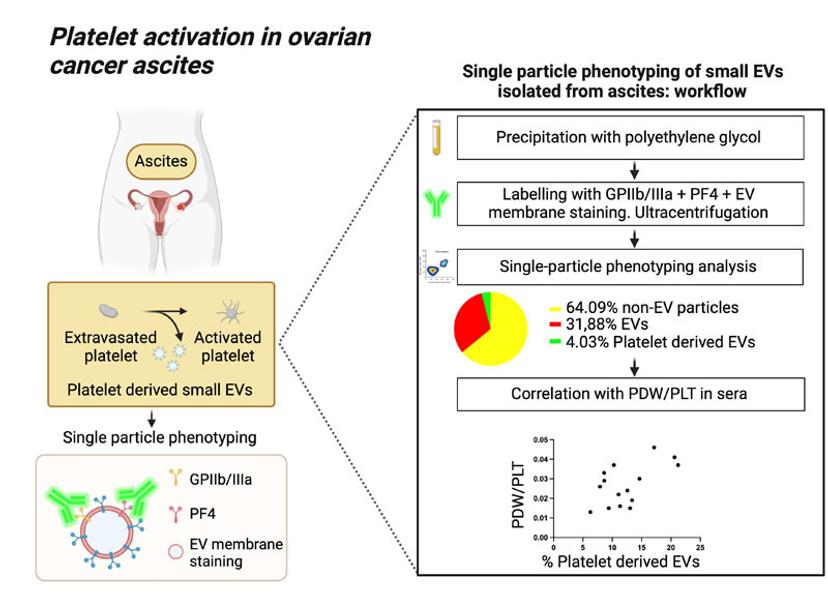
Figure 1: Bortot, B.; Mangogna, A.; Peacock, B.; Lees, R.; Valle, F.; Brucale, M.; Tassinari, S.; Romano, F.; Ricci, G.; Biffi, S. (2022). Graphical Abstract – Platelet activation in Ovarian cancer ascites [JPG]. In: Platelet Activation in Ovarian Cancer Ascites: Assessment of GPIIb/IIIa and PF4 in Small Extracellular Vesicles by Nano-Flow Cytometry Analysis. Cancers, 14(17):4100. https://doi.org/10.3390/cancers14174100
Another 2022 study, by Preusser et al., used nFCM technology to quantify EVs isolated from ascites, exploring the efficacy of the field flow fractionation technique for EV extraction.2 Using antibodies against key EV markers CD9, CD63, and CD81, Preusser was able to demonstrate the fractions with superior EV enrichment from ascites, deciphering each subpopulation individually by their fluorescent labelling.
Antibody labelling of EpCam, a biomarker of cancer stem cells, and the EV tetraspanins identified clear sub-populations in dot plots, with good separation between the fluorescent-positive and fluorescent-negative populations.
As these EVs were individually sized simultaneously for protein identification, differences in particle diameter were observed with CD9 positive EVs representing the smallest and EpCam-positive EVs being the largest (76.52nm vs 95.41nm) indicating possible differences between the tumor derived EVs and other co-isolated EVs.
EVs as nanocarriers
Not only are they promising biomarker candidates, EVs have been explored as a new class of nanocarriers for drug delivery. Significant efforts have been made to develop new techniques that would allow for the manufacture of EV-based drug formulations for the treatment of a wide range of diseases, including cancer.
By using the NanoAnalyzer, it’s possible to identify and quantify EVs carrying the therapeutic agents and simultaneously evaluate the quantity and distribution of the therapeutic agent—whether it is loaded into specific EV sub-populations related to size, co-localized proteins, and so on.
A common approach to generating EV therapeutics is to transgenically modify the host cells to produce EVs with therapeutic proteins presented on the surface or maintained on the lumen side of the EV membrane. In early-stage research, fluorescent proteins can be used as surrogates for therapeutic proteins. This allowed Silva et al to explore suitable ‘EV-sorting’ proteins for loading of therapeutic proteins fused with eGFP to EVs.3 EV isolations from transgenic HEK-293 were tested for their purity using nFCM and EV membrane labelling, identifying highly pure isolations (Figure 2b and 2c). Sub-population analysis identified four lead candidates for EV sorting, which yielded the highest proportion of loaded EVs (Figure 2d and 2e). Following this the relative quantity of loaded protein was assessed using mean fluorescence intensity (MFI). This identified a single lead candidate cell line secreting EVs with significant enrichment of the target protein (Figure 2f).
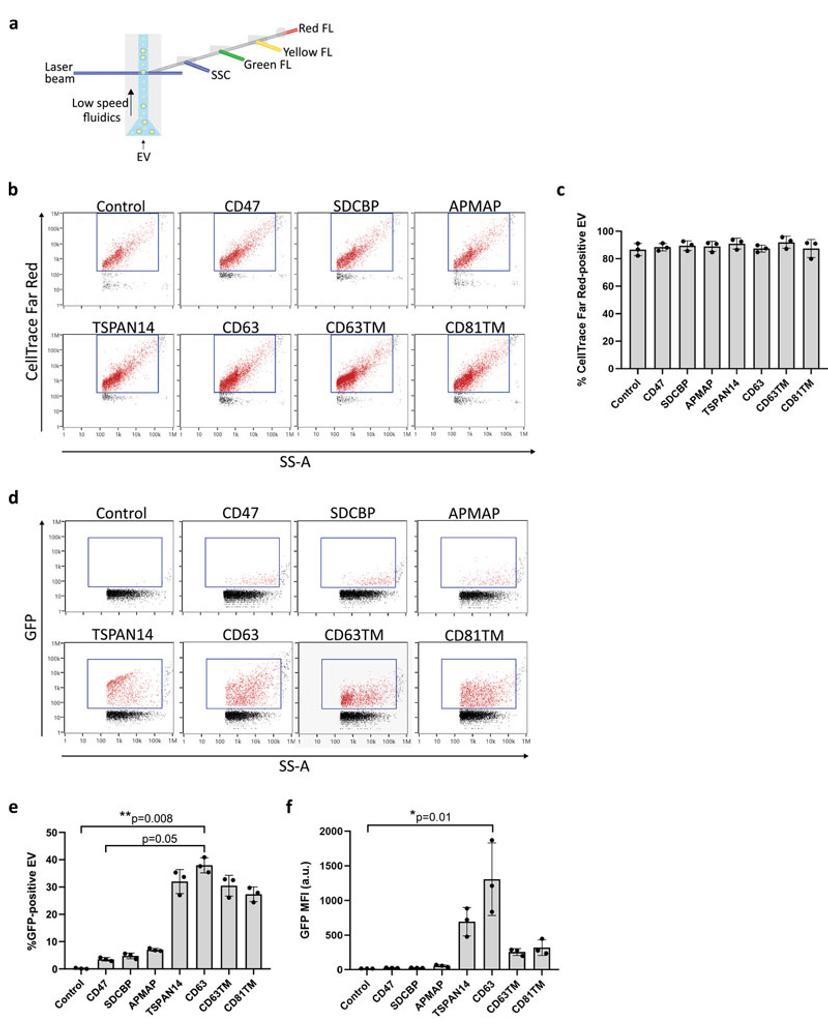
Figure 2: Silva, A. M, Lázaro-Ibáñez, E., Gunnarsson, A., Dhande, A., Daaboul, G., Peacock, B., Osteikoetxea, X., Salmond, N., Friis, K. P., Shatnyeva, O., & Dekker, N. (2021). Nanoflow cytometry analysis of Extracellular Vesicles at the single-vesicle level reveals a heterogeneous distribution of GFP among vesicle subpopulations [JPG]. In: Quantification of protein cargo loading into engineered Extracellular Vesicles at single-vesicle and single-molecule resolution. Journal of Extracellular Vesicles, 10, e12130. https://doi.org/10.1002/jev2.12130
Single EV characterization is a major driving force for EV biomarker and therapeutic development to better diagnose and treat cancer. Utilizing flexible labelling protocols, samples and products can be minimally treated before extensive characterization by the NanoAnalyzer platform.
Contact NanoFCM representatives today to better understand how nano-flow cytometry can improve your workflow either by implementing our technology locally or outsourcing through our analysis services.
References:
Bortot, B.; Mangogna, A.; Peacock, B.; Lees, R.; Valle, F.; Brucale, M.; Tassinari, S.; Romano, F.; Ricci, G.; Biffi, S. Platelet Activation in Ovarian Cancer Ascites: Assessment of GPIIb/IIIa and PF4 in Small Extracellular Vesicles by Nano-Flow Cytometry Analysis. Cancers 2022, 14, 4100. https://doi.org/10.3390/cancers14174100 Preußer, C., Stelter, K., Tertel, T., Linder, M., Helmprobst, F., Szymanski, W., Graumann, J., Giebel, B., Reinartz, S., Müller, R., Weber, G., & von Strandmann, E. P. (2022). Isolation of native EVs from primary biofluids—Free-flow electrophoresis as a novel approach to purify ascites-derived EVs. Journal of Extracellular Biology, 1, e71. https://doi.org/10.1002/jex2.71 Silva, A. M, Lázaro-Ibáñez, E., Gunnarsson, A., Dhande, A., Daaboul, G., Peacock, B., Osteikoetxea, X., Salmond, N., Friis, K. P., Shatnyeva, O., & Dekker, N. (2021). Quantification of protein cargo loading into engineered Extracellular Vesicles at single-vesicle and single-molecule resolution. Journal of Extracellular Vesicles, 10, e12130. https://doi.org/10.1002/jev2.12130
Want the latest science news straight to your inbox? Become a SelectScience member for free today>>

