Accessible, rapid testing transforms prostate cancer detection
By developing rapid, accurate, and cost-effective lateral flow tests for prostate cancer, Valley Diagnostics aims to make early diagnosis more accessible and reliable
9 May 2025
Dave Taylor, CEO, Valley Diagnostics
Prostate cancer has recently become the most common cancer in the UK, affecting more than 55,000 men every year. However, current technologies still fall short, with existing tests often complex, invasive, and prone to inaccuracies, making early diagnosis difficult. Valley Diagnostics, a medical technology company based in Wales, is determined to change this. By developing rapid, accurate, and cost-effective lateral flow tests for prostate cancer and other diseases, the company aims to make early diagnosis more accessible and reliable.
In this interview, Dave Taylor, CEO of Valley Diagnostics, shares insights into the challenges of prostate cancer testing today, the potential of novel biomarker-based LFTs, and how the company’s innovations could reshape the future of cancer diagnostics.
Explore more of the latest news, reviews, and resources in our Accelerating Cancer Research Feature here.
What is the mission of Valley Diagnostics, generally and in the oncology space?
Valley Diagnostics, based in Cardiff, Wales, is a medical technology company dedicated to transforming disease diagnostics through the development of rapid, accessible, and cost-effective testing solutions. Its mission centers on creating innovative lateral flow diagnostic tests that can detect diseases such as prostate cancer, lung cancer, and tuberculosis from urine samples.
Collaborating with Aberystwyth University, Valley Diagnostics is working to commercialize biomarkers identified through the OSCAR clinical study. These efforts are focused on developing a reliable, easy to use prostate cancer testing solution that can be integrated into national screening programs, potentially saving thousands of lives and significantly reducing healthcare costs.
Through these initiatives, Valley Diagnostics aims to make cancer diagnostics more accessible, efficient, and sustainable, ultimately contributing to better health outcomes on a global scale.
What challenges do healthcare providers and patients face when testing for prostate cancer?
Testing for prostate cancer presents several challenges for clinicians, patients and healthcare systems:
- Clinical challenges: The most common test, prostate-specific antigen (PSA), is not cancer-specific as PSA levels can be elevated for benign conditions like an enlarged prostate or infection. PSA tests can lead to false positives (unnecessary worry and procedures) or false negatives (missed cancers). The diagnostic pathways are not always clear; for example, after a PSA test, decisions about biopsies, magnetic resonance imaging (MRI) scans, or active surveillance can be complex and vary between providers or regions.
- Patient challenges: Lack of awareness or misconceptions, for example, some men are unaware of risk factors (e.g., age, family history, ethnicity – especially for black men). There is a fear of diagnosis and of treatment side effects that may cause delays in seeking testing. For many men, embarrassment or stigma related to urinary symptoms or the digital rectal exam (DRE) can discourage testing.
- Healthcare system challenges: Variation in guidelines – there is no national screening program in the UK. The UK NHS provides PSA tests for men aged 50 or over, but testing is not always actively encouraged, leading to inconsistent approaches.
What is the current industry standard for the detection of prostate cancer?
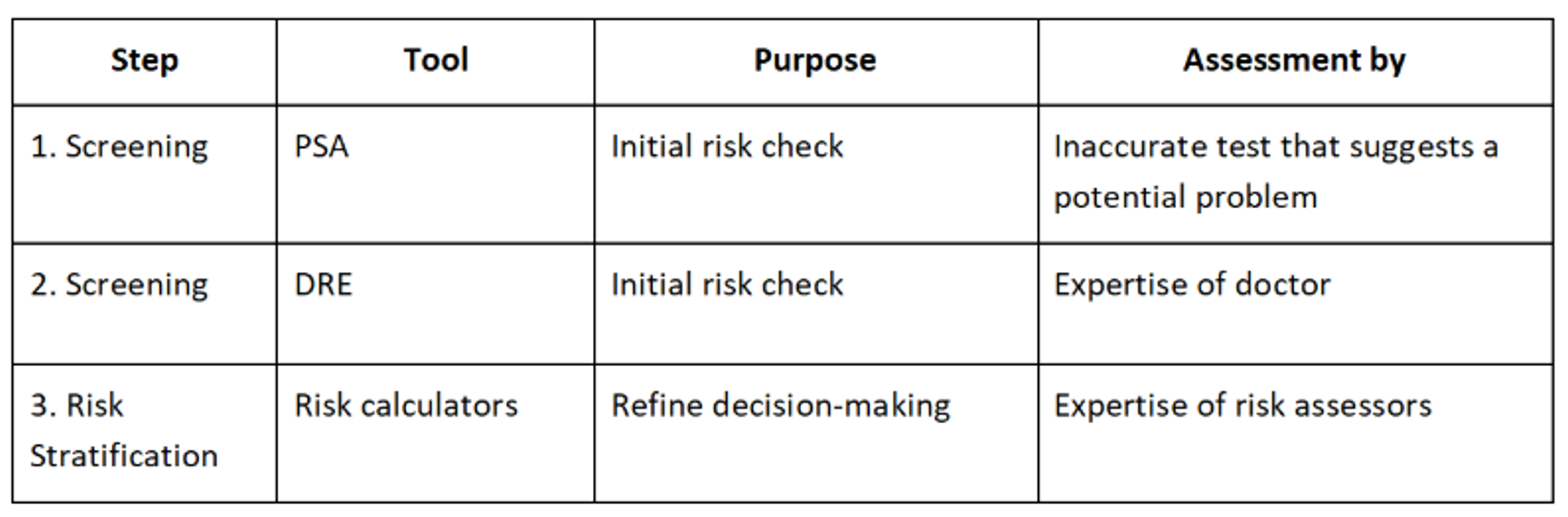
The current industry standard for the detection of prostate cancer is a multi-step approach that begins with initial screening and progresses through imaging and biopsy if warranted. This is a breakdown of the standard pathway as of now:
1. Initial screening - PSA blood test: this is the main initial test used to assess risk. PSA is a protein produced by both cancerous and non-cancerous prostate tissue. Elevated PSA levels can indicate cancer but can also frequently indicate benign conditions e.g., benign prostatic hyperplasia (BPH) or prostatitis.
2. Digital rectal exam: The doctor feels the prostate via the rectum to detect lumps, hardness, or asymmetry. This is less commonly used as a standalone but is often combined with PSA.
3. Risk assessment tools: Risk calculators (e.g., prostate cancer prevention trial risk calculator) combine PSA, DRE results, age, race, and family history to estimate cancer risk. These tools can help decide whether to proceed to more advanced diagnostics.
4. Multiparametric MRI (mpMRI): Widely regarded as the gold standard imaging method before biopsy, MRI helps to identify suspicious areas, potentially reduce unnecessary biopsies, and help guide targeted biopsies. The UK National Institute for Health and Care Excellence (NICE) guidelines now recommend mpMRI before a biopsy if PSA is elevated.
5. Prostate biopsy: Targeted biopsy (MRI-ultrasound fusion) is becoming the new standard, replacing the older 12-core transrectal ultrasound (TRUS)-guided approach. Tissue is collected and examined histologically to determine Gleason score, which grades cancer aggressiveness.
Current industry process for the detection of prostate cancer
Valley Diagnostics process for the detection of prostate cancer
What led you to developing LFTs for detecting prostate cancer?
The current PSA test-led methodology has several issues, including the following:
- Large numbers of false positives: Elevated PSA levels do not exclusively indicate cancer, leading to unnecessary anxiety and potentially invasive follow-up procedures. For instance, studies have shown that up to 75% of men with elevated PSA levels who eventually undergo biopsies do not have cancer.
- High potential for false negatives: Some prostate cancers do not produce significant PSA levels, which means the test can miss certain cancers, providing a false sense of security. Cancer Research UK acknowledges that the PSA test is not a definitive diagnostic tool for prostate cancer due to its potential for false positive and false negative results.
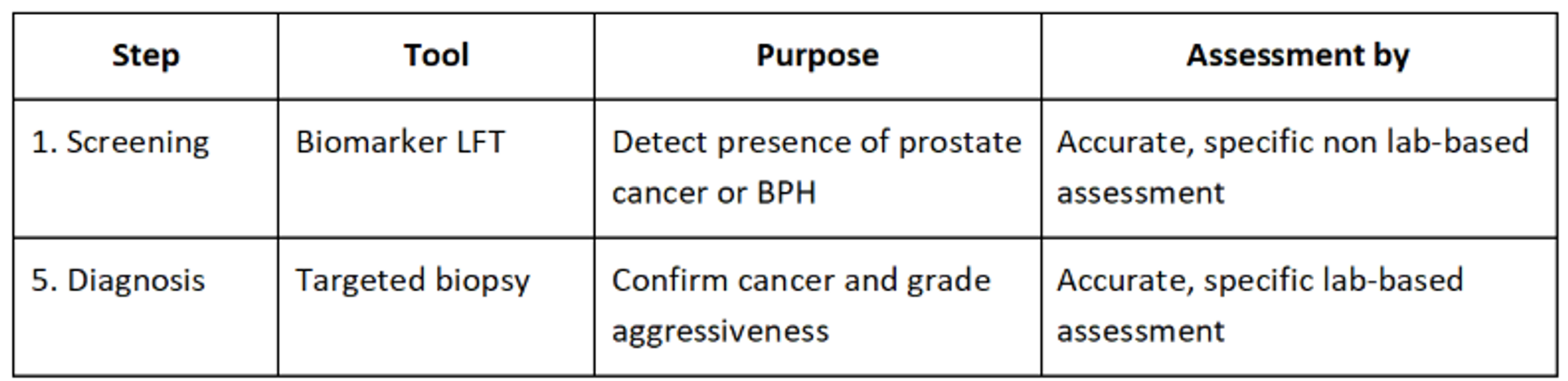
As it currently stands, a PSA test showing an elevated level of PSA in the blood will lead to a range of other tests that are both invasive and expensive. There is a better way to offer prostate cancer testing to men and the science behind biomarker identification from metabolomics and the translation to an LFT offers this.
Why are LFTs an exciting avenue for prostate cancer detection?
LFTs offer the ability to transform how, where, and when prostate cancer is diagnosed – bringing testing closer to patients in faster, cheaper, and more accessible ways.
1. Speed and simplicity: LFTs are rapid, typically delivering results in minutes. They can be used at POC settings like general practitioner (GP) clinics, pharmacies, or even at home. This eliminates the need for lab-based testing delays and repeat appointments.
2. Low cost and scalability: LFTs are inexpensive to manufacture, especially at scale. They could allow mass screening programs, particularly for higher risk populations e.g., men over 50 or black men.
3. Portability and accessibility: There is no need for specialized equipment or trained technicians. They can be deployed in rural or underserved areas where traditional diagnostics may be unavailable.
4. Expanding beyond PSA: Next-gen LFTs can be used to detect novel biomarkers, not just PSA. This will reduce the false positive rate associated with traditional PSA testing and improve specificity. For example, the Valley Diagnostics test will detect a prostate cancer biomarker fingerprint in a urine sample that determines specifically if the patient has the cancer.
5. Supports early detection: The goal is to catch aggressive cancers earlier, when they’re most treatable. Frequent, non-invasive testing could monitor changes over time, similar to blood glucose or cholesterol testing.
How do you collaborate with partners to help with their research in this space?
Valley Diagnostics works very closely with several partners to develop the tests, including a team at Aberystwyth University that is responsible for the core metabolomics research that led to the identification of the biomarker fingerprints. These biomarkers are the key to effectively detecting the presence of prostate cancer in urine samples. Valley Diagnostics has a license agreement with Aberystwyth that gives exclusive rights to develop and commercialize tests based on the biomarkers.
Aberystwyth University has been working with 12 NHS trusts in England via the OSCAR trial to collect urine samples for internal research and testing as part of the biomarker discovery program. We will be working together with these NHS partners’ samples to undertake internal verification of results. We are also working with Health Innovation North East North Cumbria and several NHS trusts in England as well as university health boards in Wales to identify suitable ways to integrate the LFT test into care pathways. We intend to use these partners in the next phase of internal verification and external validation.
Collaboration with these partners is essential in accelerating the development timelines so we can bring this important test to the market as soon as possible.
How will this work benefit patients and healthcare providers for cancer diagnoses? (What impact do you hope to have with this product/in this field?)
An easy-to-use LFT for prostate cancer detection could be a game-changer for both patients and healthcare providers, offering meaningful benefits across early diagnosis, access, efficiency, and healthcare economics.
Benefits for patients will include:
- Faster diagnosis results in minutes, rather than days or weeks. This reduces stress of waiting for results and speeds up follow-up steps.
- Easier access to testing that can be used outside of hospitals: at home, in pharmacies, or GP clinics. This will bring benefits to rural, older, or mobility-challenged individuals who may delay or skip traditional tests.
- Reduced invasiveness of testing as we are using a simple-to-use test that requires only a small urine sample.
- Increased screening rates as the simplicity and privacy of LFTs could encourage more men to get tested, especially those reluctant to visit clinics or undergo standard procedures.
- Improved equity: The simple, easy-to-use, and low-cost test reduces dependence on advanced medical infrastructure, helping underserved communities access life-saving early detection.
Benefits for healthcare providers will include:
- Better triage and prioritization: LFTs help identify high-risk patients quickly, allowing specialists to prioritize urgent cases for biopsies.
- Significantly lower burden on services as the test frees up hospital labs and imaging resources by reducing unnecessary referrals. This can help manage backlogs in urology services and cut waiting times.
- Increased cost-efficiency as LFTs are inexpensive compared to lab-based blood tests or MRIs. In addition, early detection reduces long-term treatment costs by catching cancer before it becomes aggressive.
What future innovations do you see for LFTs/POC testing for cancer?
The future of LFTs and POC cancer testing is incredibly promising, with rapid advances in biomarkers, nanotechnology, AI, and connectivity. Valley Diagnostics is already working with partners to identify biomarkers for several other cancers including breast, bladder and bowel, enabling universally accessible early-stage cancer detection.
