Addressing the Challenges of Biosimilar Analysis with NMR at the Center for Biologic Evaluation, Health Canada
Dr Yves Aubin, Research Scientist at Health Canada, spoke to SelectScience® about his research using NMR to study the structure of biosimilar molecules in the pharmaceutical industry
8 Aug 2016
Health Canada’s Center for Biologic Evaluation is responsible for providing market authorization to biologic type drugs, vaccines, hormones and monoclonal antibodies.
Dr Yves Aubin is an NMR expert who utilizes the spectroscopy technique to study the structures of protein and biomolecules. His research focuses on using NMR Spectroscopy to assess the structure of biological therapeutics, such as monoclonal antibodies.
Characterizing biosimilars
When replicating a biological molecule, such as a protein, it is challenging to ensure that “the structure of the new molecule is identical to the original molecule - that’s why we call them biosimilars”, explained Dr Aubin.
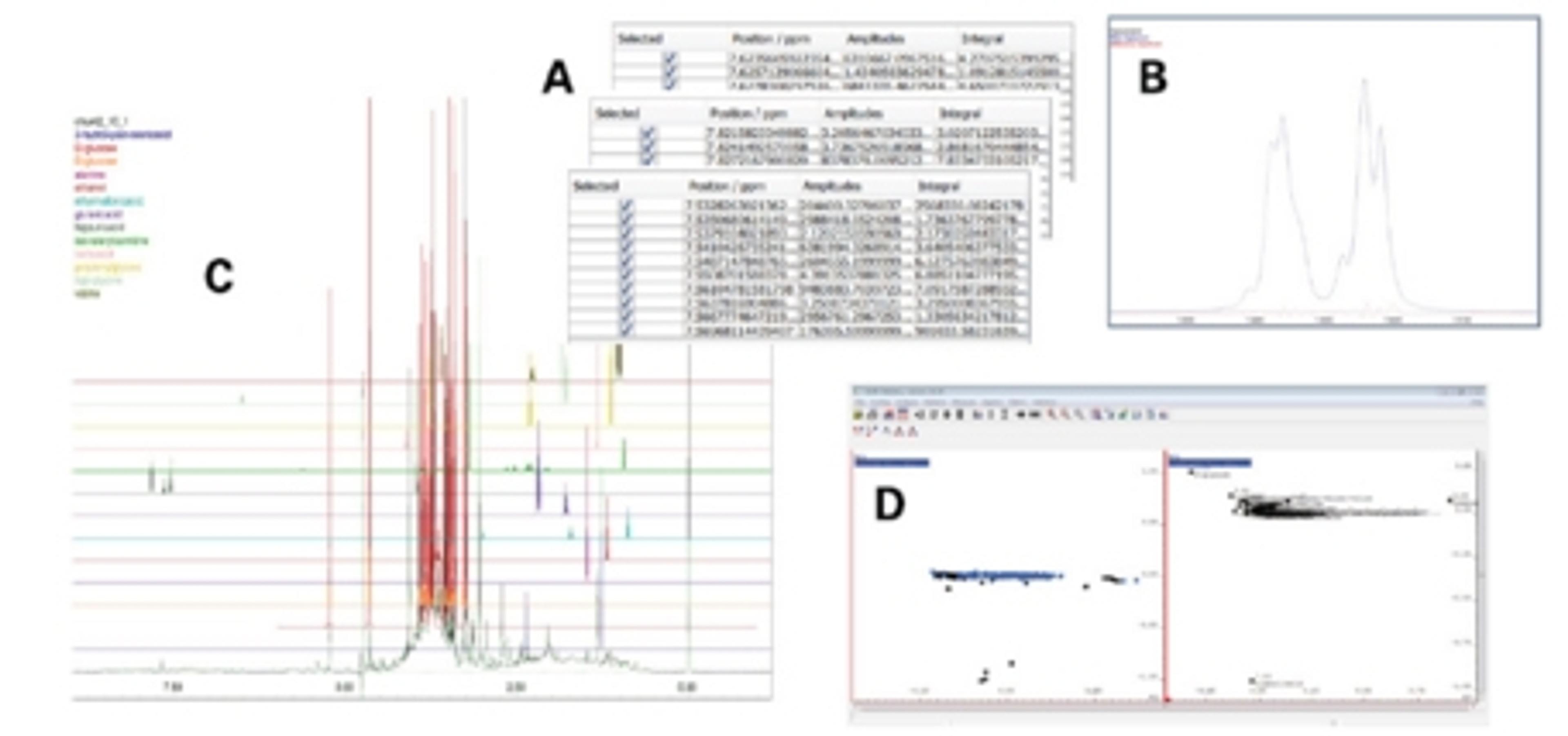
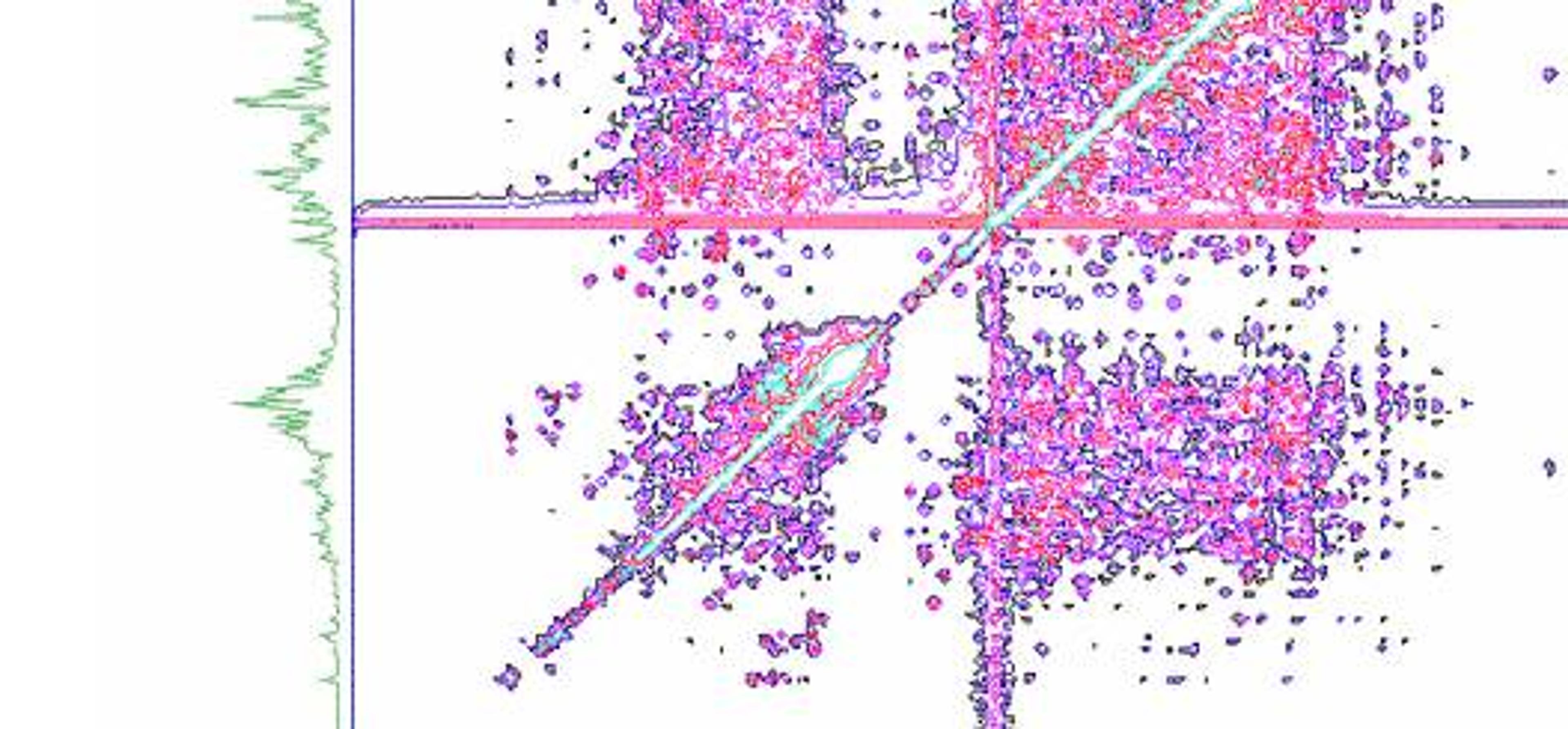
According to Dr Aubin the challenge with biopharmaceutical products is that they are very complex molecules obtained from biotechnologies. Small drug molecules like statins are straightforward to characterize because of their small size, therefore it is easy to copy them and demonstrate the similarity. Proteins are much more complex and challenging to copy, so it is more difficult to demonstrate that the composition and structure of the copy is the same or if there are variations. All regulatory agencies across the world have developed guidance for sponsors that seek market authorization of biosimilar products. The common denominator of these guidance is that a comparability exercise must conducted between their proposed product an appropriate reference product, usually the innovator product that is approved in that jurisdiction. Dr Aubin continued, “it’s not yet possible to show that a biosimilar is identical to the originator, so a number of assays need to be done. Using NMR, we can enhance the level of resolution and provide a more precise picture of these molecules. NMR fingerprints are unique to each pharmaceutical product and provide a lot of information that tells us that a biosimilar can be very similar in its structure and composition compared to the originator”. Yves explained that his team is now extending these NMR methods to monoclonal antibodies biosimilars, highlighting that “while these molecules have been very challenging to study, the recent developments in spectrometer hardware and the work of the group of Dr. John Marino at the National Institute of Standards and Technology (NIST) made it possible.” Dr Aubin also commented on their suitability as therapeutics, “antibody biosimilars are relatively quick to prepare, not so expensive to produce, and they can go in the clinic faster to demonstrate whether it is a good or a bad target and therefore obtain proof of concept much faster than with small molecules.”
Innovative NMR methods for antibody characterization
“We are collaborating with John Marino at the NIST. His group has demonstrated that it’s possible to look at antibody fragments using NMR. So we are working with them to demonstrate to the industry that the high resolution read out from these spectroscopic fingerprints, can be used to characterize the molecules.” Dr Aubin explained that the “field of antibodies NMR technology is about two years old” and highlighted the importance of this technology for the industry, “before NMR the tools researchers had to analyze the three-dimensional structure of antibodies were limited to FTIR and circular dichroism spectroscopy, which only provide a low resolution structural information, while NMR gives a high-resolution assessment, thus allowing for more detailed analysis.” Commenting on the benefits of NMR, Yves says “we have a very complex pattern that is unique to a given molecule so it provides a very high degree of complexity, from which you can compare a biosimilar product and achieve a higher degree of comparison. Both industry and regulators seek to have a more in depth characterization of these products. With the possibility of using spectroscopy like NMR that level of characterization will be achievable”
What is the future for biosimilars in therapeutics?
“We will see a lot more antibodies hitting the market for numerous diseases”, Dr Aubin explained that this is “not surprising as they can be tailored to such a number of targets.”
“Antibodies are definitely the fastest growing type of drugs in the near future, and I think the small molecule drug market will reflect that as well because the technology to make antibodies is pretty well mastered right now.”
Dr Aubin continued, “antibodies are a good ‘therapeutic’ platform to address a number of diseases whether the goal is to interfere with protein-protein interaction, such as any biological signaling, or to recruit the immune system to destroy unwanted cells such as in cancer therapies. Using NMR as an accurate and reliable means to characterize antibody based therapies should help enable and potentially accelerate, public access to novel treatments that are both safe and effective.”