Advances in Lateral Flow Technology for the Point-of-Care Market
Current technological advances are broadening the diagnostic possibilities for lateral flow platforms
30 Oct 2015
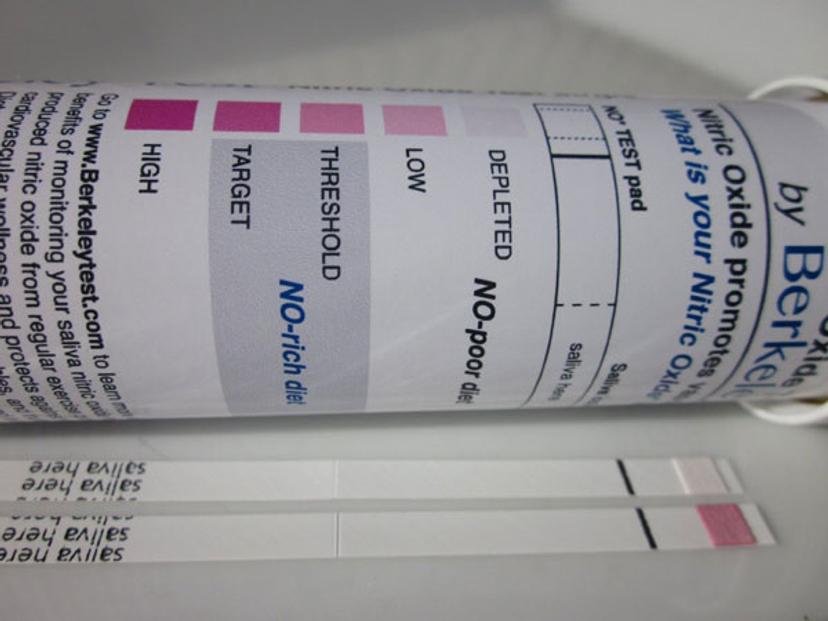
Lateral flow assays, such as this saliva test for nitric oxide, are a mainstay technological platform in the IVD industry
Lateral flow assays are a well established, cost-effective technology designed to detect target analytes in a sample. Lateral flow immunoassays, also known as immunochromatography assays, are simple assays that all use the same basic principle.
Each assay typically consists of a sample pad, on to which the test sample is applied; a conjugate pad containing the target analyte conjugated to coloured particles (usually gold nanoparticles or latex spheres); the reaction membrane, on to which a line of anti-target analyte antibodies are attached; and an absorbent waste pad that pulls the sample across the reaction membrane.
Ease of use
Lateral flow immunoassays have the advantage of being easy to use; they can test small sample volumes, they usually have a long shelf-life, they can produce results quickly and can have high specificity and sensitivity.
Disadvantages of lateral flow assays include a difficulty in multiplexing (simultaneous marker analysis), some sensitivity issues and challenges in test-to-test reproducibility.
The assays are relatively low cost to produce and have a short timeline for test development and market approval. As a result, they are a popular platform of choice for developers of rapid disease diagnostics. These rapid diagnostic tests may be used by laboratory personnel, but often the ultimate goal is to move the testing outside of the laboratory, to the point-of-care.
Recent advances
Advances are being made in the application of lateral flow assays to multiplex testing. In September 2015, SCIENION AG and Axxin Pty Ltd announced a partnership to collaborate. The alliance brings together SCIENION's picoliter dispensing technology and Axxin's experience in commercializing diagnostic instrument systems for point-of-care diagnostics. Initial beta testing of the new platform has proved promising and the product in development will be capable of analyzing up to 20 parameters for infectious diseases, drugs of abuse and allergies.
It is even possible to screen for myeloma now using lateral flow technology. Abingdon Health markets a rapid diagnostic device called Seralite® that uses monoclonal antibodies conjugated to gold particles, incorporated into a hand-held lateral flow device. The platform is able to provide a quantitative measurement of kappa and lambda free light chains to support the diagnosis and monitoring of myeloma.
As the demand for more sensitive and faster assays increases, lateral flow developers have increasingly sophisticated technology at their disposal. Anteo Techologies produces a 200nm Coupling Kit that makes conducting lateral flow tests and biomolecule separation easier and more flexible. Anteo Technologies states that magnetic particles are easier to handle than gold particles and that they also have greater sensitivity.
The 200 nm Coupling Kit makes conducting lateral flow tests and biomolecule separation (including cell separation) easier and more flexible
Earlier this year, NanoHybrids announced the release of a new line of spherical gold nanoparticles - the coloured particles conjugated to the target analyte on the conjugate pad. Using a proprietary synthesis method, these gold nanoparticles are produced at a 10-15x higher concentration as compared to traditional protocols. NanoHybrids claims that the new monodisperse gold particles enable the development of assays that reach the highest specifications with regards to sensitivity and reproducibility.
Aptamers are also gaining increasing attention for use in lateral flow tests. They have an almost unlimited shelf-life, they can be selected to bind difficult targets and they allow for very well-defined, single-site conjugation chemistries. Download this application note from Base Pair Technologies to learn more.
Lateral flow assays are a mainstay of the in vitro diagnostics rapid testing industry. Recent advances are helping to really optimize these tests for use in the point-of-care market.
Image credit: "Saliva test for nitric oxide" by Teststrips - Photo of test strips in my home. Licensed under CC BY-SA 3.0 via Wikipedia - https://en.wikipedia.org/wiki/File:Saliva_test_for_nitric_oxide.jpg#/media/File:Saliva_test_for_nitric_oxide.jpg

