Aptamers to Detect Small Traces of Food Allergens
The combination of aptamers and recombinase polymerase amplification (RPA) enables quick, accurate detection of allergens
17 Oct 2018
The emboldened words appearing on a food label emphasizing the presence of allergens can, for some individuals, influence their experience at the food table. In addition to caloric value and nutrition, drawing attention to food allergens such as wheat, milk, gluten, fish and nuts, among others, is mandated by food regulators across the world. On the consumer’s side, a simple phrase such as ‘may contain nuts’ can inform a mother when shopping for her child. For the food manufacturer, on the other hand, there lies a big responsibility in ensuring the presence of these allergens – even in nanomolar concentrations.

In this SelectScience® interview, we speak with Dr. Ciara O’ Sullivan, ICREA Research Professor at the Universitat Rovira i Virgili, who has a clear goal: being able to detect all known food allergens using a simple paper test.
We are the first group to combine food allergen detection with recombinase polymerase amplification (RPA) technology.
Dr. Ciara O' Sullivan ICREA, Universitat Rovira i Virgili
The key to detecting traces of allergens buried in complex food materials is the sensitivity of the test used. “We are the first group to combine food allergen detection with recombinase polymerase amplification (RPA) technology,” says O’ Sullivan.
O' Sullivan made two choices that enabled a speedy, accurate detection of trace allergens. The first choice: the use of aptamers or nucleic acids to bind to allergens. “We’ve developed aptamers which are nucleic acid analogs of antibodies,” Sullivan explains. “They work as well, or even better than antibodies: they bind with high affinity and high specificity with the targets, which are, in this case, the allergens.”
The calculated choice of aptamers (nucleic acids) over antibodies (proteins) in O' Sullivan’s experimental design imparts high sensitivity because nucleic acids can be amplified, and therefore, trace amounts of allergens can be detected. O' Sullivan continues: “We can have a capture aptamer that binds to the allergen. The secondary aptamer then binds to the allergen, its amount proportional to the amount of allergen bound.” In a typical ELISA, the secondary aptamer or antibody would have an enzyme label. In O' Sullivan’s case, the secondary aptamer, being a nucleic acid, can be amplified using a polymerase, thereby amplifying the data of detected allergens.
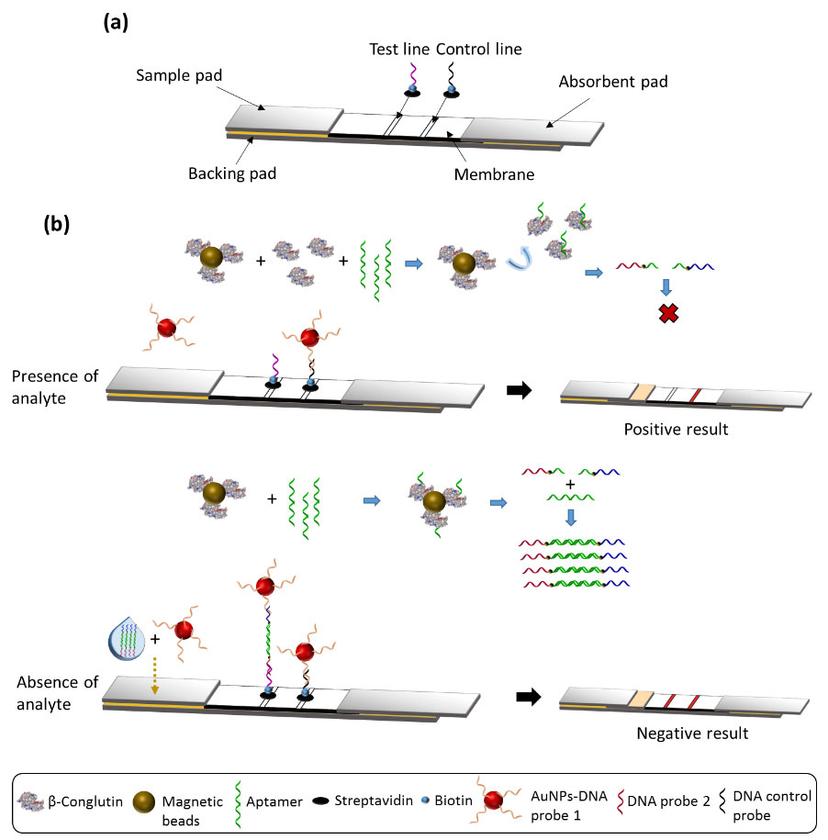
A schematic representing the paper test developed by the O' Sullivan lab to detect the presence of food allergens. A combination of aptamer binding and RPA technology makes this test accurate and suitable for in situ applications. Image courtesy of the O' Sullivan lab.
This brings us to the second informed choice O' Sullivan and her team made in their experiments – to use the RPA technology. RPA, the brainchild of TwistDx, amplifies nucleic acids at a single temperature, as against PCR which requires thermal cycling, and is much quicker, yielding results in only 3 – 10 minutes, as compared to the conventional overnight PCR experiments. To speed up allergen detection, O' Sullivan’s lab coupled the aptamer binding experiments with RPA.
We want to be able detect allergens in situ, at the point of need.”
Dr. Ciara O' Sullivan ICREA, Universitat Rovira i Virgili
“We combine allergen binding with RPA, i.e. we use the secondary aptamer as a template for RPA. We then carry out the RPA reaction using specially designed tailed primers – this enables a direct detection of RPA amplicons via hybridization. We then quantify the products of amplification using either an enzyme-linked oligonucleotide assay or lateral flow assays,” O' Sullivan says. “So, now we have a combination of aptamer detection, which accurately binds to the specific allergen, and RPA, which amplifies the detection, working together to give us very low femtomolar detection limits of allergens.”
“So far, we have aptamers against the food allergens gliadin and β-conglutin. Susceptible individuals can get an anaphylactic shock following exposure, inhalation or ingestion of β-conglutin found in lupin flour and products. There have even been reports of deaths from this,” O' Sullivan notes. “We’re working on expanding to have a panel of 14 different food allergens whose presence in food products must be labeled according to both EU and US regulations.”
“As for the nucleic acid aptamers, once you’ve selected them, the production process costs a fraction of that of an antibody. And because it’s nucleic acid, it’s much more stable over long periods of time and doesn’t need refrigeration,” O' Sullivan adds. “Also, RPA is so robust and can even work at room or body temperature.” This would make the test much more suitable for on-site measurements at food factories instead of limiting the testing to labs.
“PCR is inherently laboratory-based. We have previously worked with collaborators to develop microsystems using PCR, but the thermal cycling requirements really push up the costs and the complexity,” says O' Sullivan. “TwistDx facilitates the use of RPA at any site. It works at room temperature and is stable as it comes in lyophilized pellets.”
“Our focus is to move out of the laboratory,” O' Sullivan concludes. “ We want to be able to detect allergens in situ, at the point of need such as at food processing plants or even by consumers.”