Behind-the-scenes at Coca-Cola: The key to high-quality drinks and happy consumers
Learn how microbiological quality control tests at Coca-Cola ensure consumer safety
26 Nov 2020

Popping a can of soda may only take a second. But behind each can or bottle of soft drink is a long, highly regulated quality control process that maintains beverage integrity and protects consumer health. In this exclusive SelectScience interview, we get a sneak peek behind the scenes at Coca-Cola and learn more about how quality is maintained throughout the manufacturing process.
“I've been at Coca-Cola, Mexico, for about five years now. I started as an intern and then advanced to a microbiology specialist, and now technical field operations manager,” states Zazil Sosa. With stringent governance on the quality and safety of its products, Sosa is responsible for the implementation of Coca-Cola’s technical strategies across the country: “I provide technical support for all of our regions in Mexico and ensure that these quality control protocols get implemented.”
The sugar content, preservatives, and short shelf-life make soft drinks susceptible to spoilage. Quality control checks, performed at different steps along the manufacturing process, increase the likelihood of detecting even the slightest microbial growth. “We have to review and ensure that all the filling lines in the bottling systems are in optimal condition to prevent and mitigate any health risks to our consumers. We also need to maintain high regulatory standards for all international food authorities,” notes Sosa.
As beverages are not sterile, having microbiological checks in place upholds the safety of the product for human consumption. “We have quality, safety, and environmental programs in place to make sure our products meet high standards. We’ve developed key indicators to check if there are any issues in the finished product or along the manufacturing lines,” says Sosa. “If we see something – even a tendency for microbiological activity – we immediately take corrective action.”
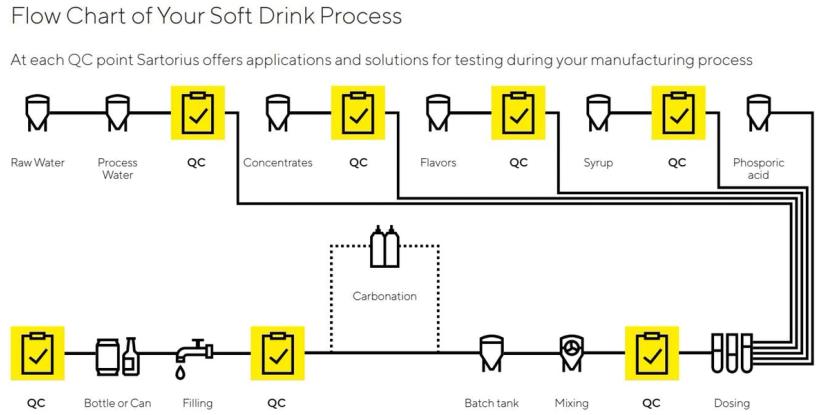
In Mexico, businesses that own beverage bottling plants purchase the proprietary concentrates from Coca-Cola. Once the bottlers prepare the beverage by adding water, flavoring, carbonation, and other ingredients to the concentrate, the final products are available for sale. However, along every step of the process, each of these 63 bottling plants must perform quality testing on the in-process and finalized product.
At Coca-Cola, quality checks involve evaluating, analyzing, and generating reports for its ready-to-drink beverages portfolio through various techniques that assure its compliance with the food safety standards. “One of the most common procedures to analyze the microbiology of beverages is the membrane filtration technique,” says Jessica Benítez, a microbiology analyst in Sosa’s team. “We filter the liquid through a nitrocellulose membrane with 0.45μm porosity, retaining any microorganisms present. Then, this membrane is placed over culture media and incubated at a specific temperature based on the target microbes. The final test is to observe and measure any microbial growth from these membranes,” Benítez explains.
Facilitating quality control protocols requires starting materials that are of high quality themselves. “Sartorius offers great quality materials in its membrane filters. The filters are always tested so we can be confident that they are sterile and ready to use,” says Benítez. “They also contain an extra layer of protection, so we can handle the membranes properly without the fear of cross-contaminating our culture media.” To perform these quality controls, the team uses Sartorius sterile cellulose nitrate membrane filters for microfiltration tests. “We receive a lot of samples from different plants in the country. So, we use these membranes daily in our laboratory,” says Sosa. Benítez adds: “Every time we need to evaluate carbonated drinks, bottled water, or any other beverage that lacks pulp or gums, we use these membrane filters by Sartorius to make sure that our products are free of pathogenic and non-pathogenic microbes.”
In conjunction with the membrane filters, the team also uses Picus® Electronic Pipettes to carry out microbial activity tests. Describing the technical must-haves in the lab, Benítez says: “Sartorius pipettes are among my top three lab essentials because of their accuracy and ease of use, followed by our microscope where we can visualize the microorganisms we are dealing with, and finally, of course, our incubators that provide the appropriate temperature for microbial growth.”
With consumer health at the core of these tests, the fast-paced manufacturing line at Coca-Cola is closely monitored, carefully controlled, and regularly optimized to meet evolving safety standards. In light of the recent pandemic, adopting virtual and digital processes may become the new normal at bottling plants. Sosa adds: “To make quality control processes even more efficient, I anticipate that we would have more rapid tests in the future, giving us detailed information about our products that we can then digitally integrate for record-keeping and data analysis.”
Learn more about how Sartorius can support you with your soft drink QC processes >>

