Biological Mechanisms Involved in Aging and Degenerative Diseases
How understanding the mechanisms involved in cellular aging and degeneration can help accelerate drug discovery
10 Jun 2018
Dr. Emilie Bureau is a leading scientist from the Compound Profiling and Cellular Pharmacology group at LifeArc, specializing in developing cell-based assays for use in the study of aging and neurodegeneration, with the aim of helping to advance drug discovery in oncology and neuroscience. She is currently working on two exciting neuroscience projects and is due to present a fascinating session on her latest findings at SLAS Europe 2018 in Brussels at the end of June, where she will be discussing her work on the TDP-43 protein, and its role in cellular aging and degeneration. In this interview, SelectScience speaks to Dr. Bureau about her research, in anticipation of her SLAS presentation.

SS: Please introduce yourself and your role
EB: I graduated from the European School for Biotechnology of Strasbourg (ESBS), France in 2004 then came over to London to do my Ph.D. at Cancer Research UK (CRUK). After graduating from University College London (UCL) in 2008, I joined the Compound Profiling and Cellular Pharmacology group at LifeArc (formerly MRC Technology), where I am a scientist developing cell-based assays for a variety of small molecule and therapeutic antibody projects mainly in the fields of oncology and neuroscience.
SS: Tell us about the research you are carrying out at LifeArc
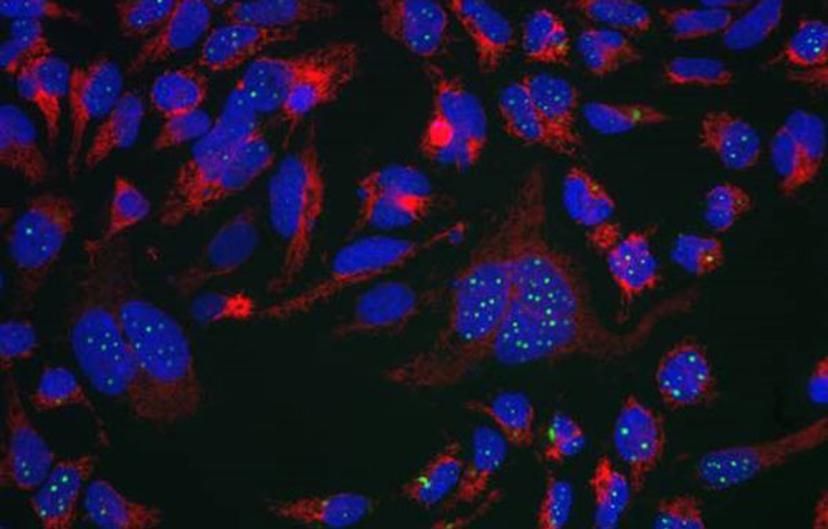
EB: For the past seven years, I have mostly been involved in developing immunocytochemistry and live cell imaging assays. I am currently working on two neuroscience projects. I am the lead scientist on a high content target-agnostic phenotypic screening program based around TAR-DNA binding protein 43 (TDP-43), a protein which is involved in amyotrophic lateral sclerosis (ALS) and other neurodegenerative diseases. This project is run in collaboration with Dr. Marco Baralle at ICGEB, Trieste, Italy and is funded by ARUK’s Dementia Consortium fund. The aim of the project is to identify compounds which induce TDP-43 aggregate clearance at the phenotypic level regardless of their mode of action. Characterizing how these compounds work may help with the understanding of TDP-43 proteinopathies. In parallel, I have been working on understanding the roles of plexin and semaphorins in multiple sclerosis (MS).
SS: How is LifeArc pioneering new ways to turn science into greater patient impact?
EB: As a charity, LifeArc aims to change the lives of patients by harnessing the potential of science. Lifearc is doing so by working in collaboration with academic, biotech and pharma partners concentrating on four specific medical areas: neuroscience, antimicrobial, immuno-oncology and respiratory. Each research program is tailored to the needs of a given project and milestones are reviewed regularly with constant dialogue and exchange of ideas with our collaborators.
This year, LifeArc is also opening two new funding models to invest in medicines, a Philanthropic and Seed fund. The Philanthropic Fund will provide grants for academic research on rare diseases and the Seed Fund will invest in promising early stage therapeutics and help progress these towards the clinic.
SS: Which technologies are causing the most innovation in your field?
EB: Development of cell co-cultures or 3D cultures of brain cells derived from human iPSCs are promising technologies to obtain more physiologically relevant neurodegenerative disease models in a dish. Recent achievements in obtaining iPSCs from patients or to be able to engraft brain organoids into rodents also highlight promising avenues.
SS: Tell us about the session you will be presenting at SLAS
EB: I will be presenting my work on TDP-43 in the “Biological Mechanisms Involved in Aging and Degenerative Diseases” session. This session will focus on how understanding the molecular basis of the different mechanisms involved in cellular aging and degeneration can be used to discover drugs targeting these pathways.
SS: What do you see for the future of your research?
EB: We have identified compounds which decrease TDP-43 aggregation, decrease sequestration of endogenous TDP-43 and therefore recover splicing function of TDP-43. Proof of concept studies are currently ongoing, investigating the potential of these compounds to rescue locomotive defects in a TDP-43 Drosophila model. Future work will aim to identify the targets mediating this response and will lead to a more directed drug discovery project.
Catch Emilie Bureau's presentation at SLAS Europe 2018 in the Emerging Investigative Biology session at 2pm on Wednesday, June 27
Visit SelectScience booth #414 at SLAS Europe, June 27-29, to meet the team and pick up a free gift.
