Biosimilar development: Experts discuss analytical characterization toolkits for antibody therapeutics
Experts from Xbrane Biopharma discuss the biosimilar development process and how bioassays are the driving force behind reduced cost and accelerated timelines
2 Feb 2023

Dr. Niklas Finnberg, Head of Non-clinical, and Dr. Dorota Klaczkowska, Non-clinical Senior Scientist, Xbrane Biopharma
With many of the existing patents of monoclonal antibody blockbuster drugs set to expire in the next few years, biosimilar development has become increasingly relevant in the biopharmaceutical space. A biosimilar is a biologic that has no clinically meaningful differences concerning safety and efficacy to an original therapeutic, or reference product1. As part of biosimilar development, there is a rigorous evaluation process to prove comparability of the biosimilar to the reference product and ensure the safety, effectiveness, and quality of the biological product2.
The biosimilar development process bears a host of development and analytical challenges and regulatory considerations to surpass before achieving market authorization. Xbrane Biopharma AB, situated in Sweden, is one of four companies in Europe dedicated to the development of biosimilars. In this article, experts, Dr. Niklas Finnberg, Head of Non-clinical, and Dr. Dorota Klaczkowska, Non-clinical Senior Scientist, at Xbrane, discuss the biosimilar development process, the integral role of bioassays in their work, and the potential of biosimilars to drive a future of more equitable healthcare systems.
The leading edge of biosimilar development at Xbrane Biopharma
Finnberg oversees and establishes bioassay and clinical bioanalytical (e.g., pharmacokinetics (PK), anti-drug antibody (ADA), neutralizing antibody (NAb)) assay programs that support the analytical and clinical pharmacology efforts of Xbrane. As a senior scientist within the Non-clinical team, Klaczkowska’s primary responsibility is to develop specific non-clinical assays that will help characterize the company’s biosimilar candidates in relation to the reference product. Together, Finnberg and Klaczkowska face the challenges of identifying the most appropriate bioassays reflective of the reference product mechanism of action that will, with sufficient granularity, characterize Xbrane’s biosimilars during the program’s preclinical phase. Furthermore, the Non-clinical team is responsible for establishing validated pharmacokinetics and immunogenicity assays in support of the biosimilar development program during its clinical phase.
Towards the end of 2022, the European Commission granted marketing authorization for Ximluci® (ranibizumab), a biosimilar candidate developed by Xbrane and its partner STADA referencing Lucentis®, an anti-vascular endothelial growth factor (anti-VEGF) for the treatment of retinal vascular disorders – a leading cause of blindness globally3. Earlier this year, Ximluci® was also approved in Great Britain, with an anticipated FDA submission during the first quarter of 2023. This is one of several therapeutics that Xbrane has in its development pipeline, which covers health concerns of considerable societal costs worldwide.
Current biologics are expensive and have a high cost to society.
Dr. Niklas Finnberg Xbrane Biopharma
Klaczkowska explains that “the mission of Xbrane is to make drugs more affordable to increase access to drugs across the human population.” Finnberg elaborates, “current biologics are expensive and have a high cost to society. So, we want to lower the cost and increase access to these treatments.” He continues, “the company’s primary focus is to develop biosimilars for autoimmune indications and oncology indications.”
The use of a patented technology lowers the overall cost of Xbrane’s biosimilar development process. Finnberg explains that this technology gives the company a competitive edge as there is no need to compromise on the quality of the expressed protein. Klaczkowska goes on to say that the technology also allows them to produce the best possible yield with good quality. Therefore, the overall production cost might be reduced.
Overcoming the analytical and development challenges faced in biosimilar development
Biosimilar development is a complex process with several regulatory hurdles and challenges to overcome. Finnberg explains, “we need to make sure we can say with certainty that there's no difference with respect to efficacy and safety of our biosimilar compared to the reference product. We want to do that early on, at the preclinical stage where we work with bioassays to characterize the structural biochemistry. The aim is to identify any differences in the activity of our biosimilar compared to the reference product before we reach the point of where we take it to the clinical trials. Hopefully, that will be enough to convince regulators that we have a sufficiently similar product, with respect to efficacy and safety.”
Iurii Bukhta @ 123RF.com
To prove biosimilarity, the group carries out a number of comprehensive laboratory state-of-the-art orthogonal analytical methods. “Initially, we must show that our product has a highly similar structure, potency, binding properties, and purity. Then, the next stage is proving equivalent efficacy and comparable safety in clinical trials with clinically relevant analytical methods,” Klaczkowska details.
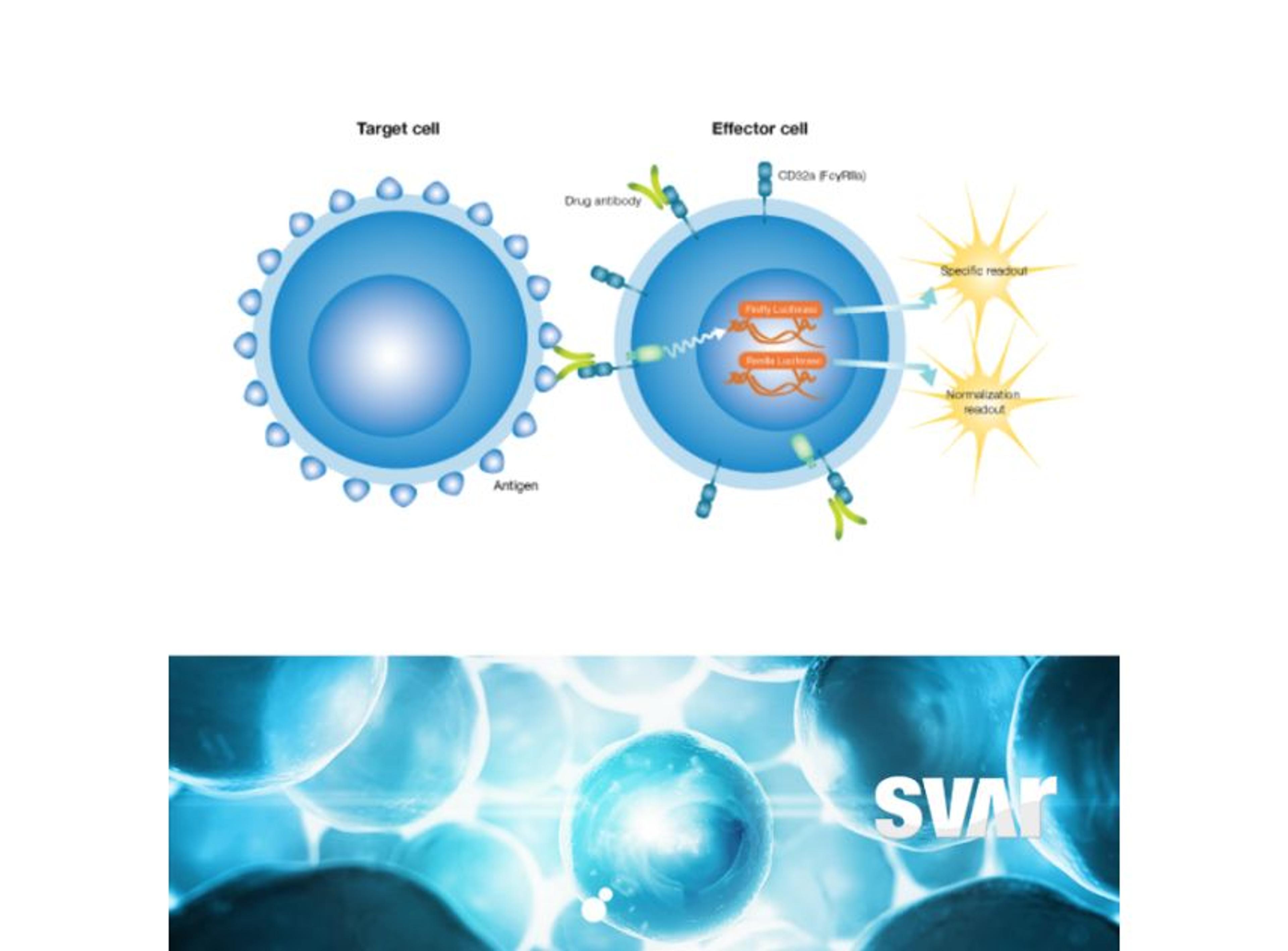
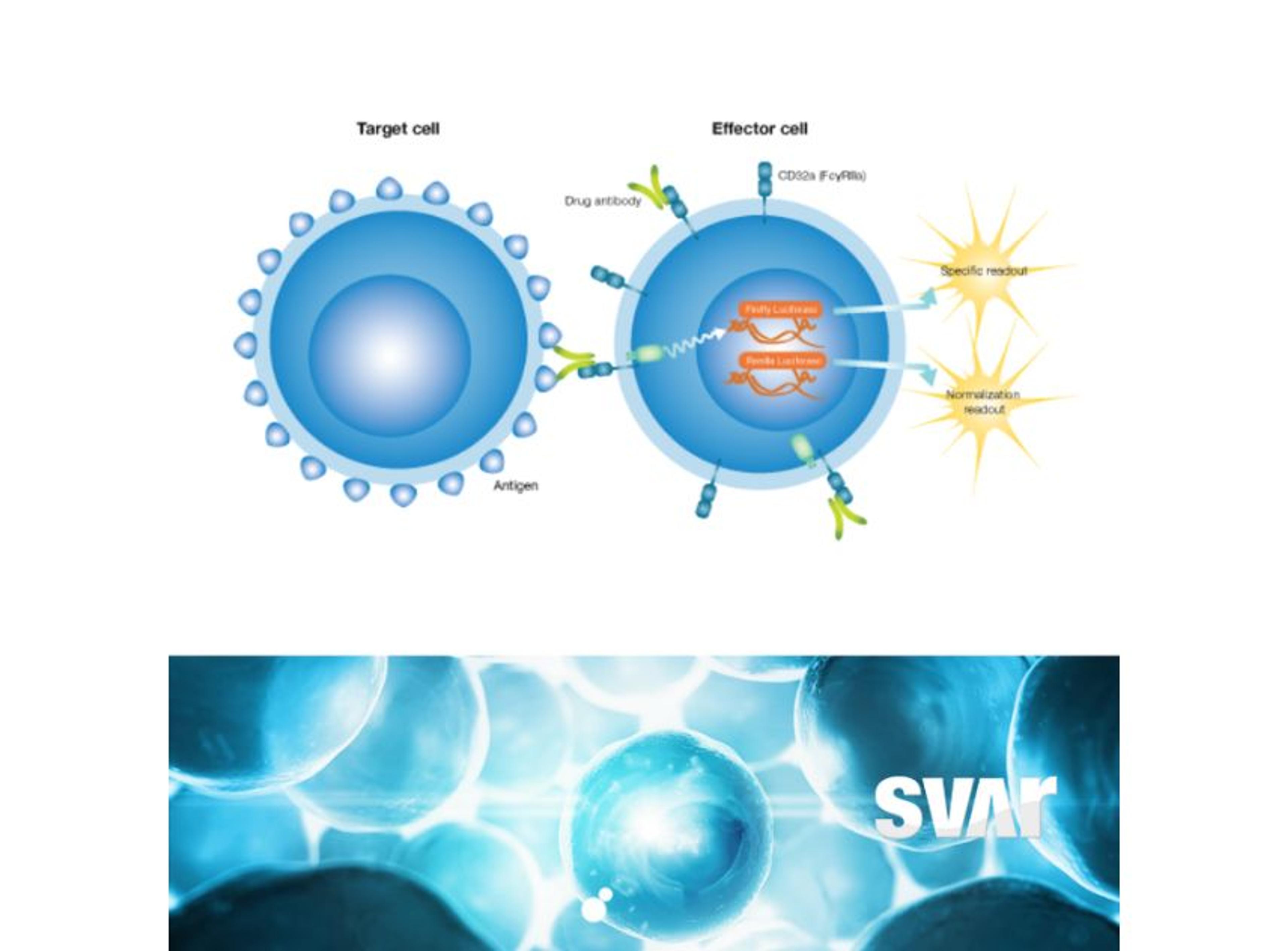
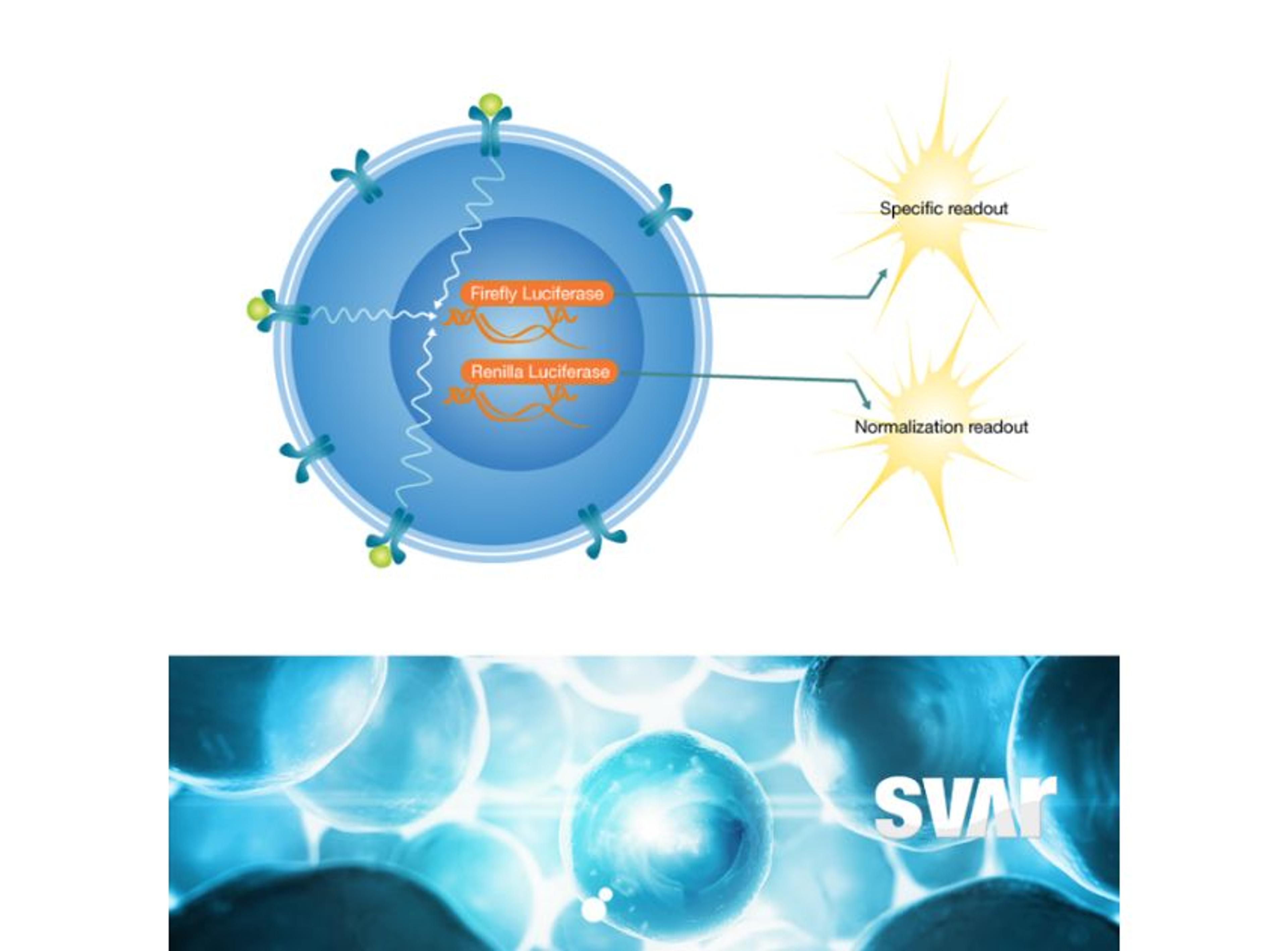
The mode of action of the drug is a very important consideration and the right analytical methods must be chosen from the beginning to accurately show the similarity. “The main focus is on physicochemical and biological characteristics of the antibodies, including bioactivity and binding of the antibody to the target. We look very closely at the fragment antigen-binding (Fab) region and fragment crystallizable (Fc) region-related activities as these influence the functionality and mode of action of the antibody,” Klaczkowska explains. To determine if binding is functional – either inhibiting or enhancing the target – the team characterizes antibody-dependent cell-mediated cytotoxicity (ADCC), the Fc-mediated activity of some IgG1 monoclonal antibodies that trigger the immune system to kill cells.
Robust analytical methods are key to proving biosimilarity
We use cell-based, functional assays in every step of the drug development process.
Dr. Dorota Klaczkowska Xbrane Biopharma
High-quality bioassays are of value to the Xbrane team throughout the development process. Klaczkowska explains, “at the center of our analytical processes is looking at the binding and potency of our drug. Additionally, I work with methods that are used for clinical bioanalysis such as ADA, NAb, and PK assays that are used in the clinical trials. So, it’s important to have good bioassays in place as they are needed for characterization of our biosimilar product.”
Finnberg highlights the increasing importance of the preclinical analytical package in biosimilar development. “We see an increase in reliance on the analytical package, of which bioassays are a part. This is because we are aiming to move towards a reduction in the reliance on clinical efficacy trials to get biosimilars approved. For example, the Medicines and Healthcare products Regulatory Agency (MHRA) has taken some assertive steps to circumvent the need for efficacy trials for biosimilars. The flip side of that is, of course, that would increase the importance of the analytical package at the preclinical stage. You need to make sure that you have bioassays that are truly reflective of the mechanism of action of your compound in patients in vivo.”
Svar Life Science has very well-defined bioassays, from which we achieve very reliable results.
Dr. Niklas Finnberg Xbrane Biopharma
Xbrane collaborates with Svar Life Science as a provider of bioassays that produce reliable and trustworthy results. Finnberg explains “the assays that you use to assess the activity of your antibody are an important consideration. Svar Life Science has very well-defined bioassays, from which we achieve very reliable results.”
The bioassays, which come in a user friendly ready-to-use format, have been utilized across Xbrane’s biosimilar development process. Klaczkowska describes, “we use cell-based, functional assays in every step of the drug development process. From the very beginning, in preclinical phase, to lot release testing. We depend on these assays, so they are used to ensure quality of manufacturing processes and they are essential for the drug product as they can provide critical data on stability and potency. We also use ADCC and ADCP bioassays from Svar Life Science in early-stage development to characterize and rank our IgG1 antibody candidates.”
The impact of biosimilars in the future of healthcare
The development of biosimilars holds promise to serve as a driving force to achieve more sustainable and equitable healthcare by bringing competition to the marketplace4. “Globally, the development of biosimilars is growing since many originators are facing the patent expiry. I think this is an exciting prospect because it will make drugs more affordable and allow better opportunities in terms of treatment for patients that are unable to afford very expensive drugs right now,” Klaczkowska shares.
As mentioned in this article, a focus has been placed on reducing the reliance on phase three or efficacy clinical trials, with the aim to reduce the cost of biosimilar development in the future. In turn, this places increasing importance on the bioassays used during preclinical development. Bringing the development cost of biosimilars down may further drive down the market value of many therapeutics, increasing accessibility of the drugs to underserved minorities.
References
Center for Drug Evaluation and Research (12 December 2022), Review and approval, U.S. Food and Drug Administration. Available at: https://www.fda.gov/drugs/biosimilars/review-and-approval#approval%20process
Guidelines on evaluation of Biosimilars (22 April 2022), World Health Organization. Available at: https://www.who.int/publications/m/item/guidelines-on-evaluation-of-biosimilars
Stada and Xbrane secure E.U. approval for Ximluci® (ranibizumab) biosimilar referencing Lucentis® (November 2022), Xbrane Biopharma. Available at: https://xbrane.com/en/mfn_news/stada-and-xbrane-secure-eu-approval-for-ximluci-ranibizumab-biosimilar-referencing-lucentis/
de Mora, F. 2019. Biosimilars: A value proposition, BioDrugs, 33(4), 353–356. Available at: https://doi.org/10.1007/s40259-019-00360-7