Cell therapy: A GMP-compatible solution for ensuring clonality during iPSC manufacturing
The journey to the VIPS™ PRO single-cell seeding platform
25 Sept 2022
There is growing demand for cell-based therapies such as chimeric antigen receptor (CAR) T-cell therapy to treat cancer and other diseases. Within the past few years, allogenic cell therapies have started to emerge enabling patients to be treated with mass-produced cells much more quickly and at a fraction of the cost of older patient-specific cell therapies. Typically, allogenic cell therapies are developed by gene editing single induced pluripotent stem cells (iPSCs) using a process known as single-cell cloning to produce other types of body cells with the desired characteristics. As with the development of any therapy, it is vital to have processes in place to ensure the final cell product is high-quality, well-qualified, and reproducible. Manual single cell dispensing methods such as limiting dilution are labor-intensive and prone to human error, therefore there is a need for automated solutions that are compatible with good manufacturing practice (GMP).

In 2017, Solentim – now part of Advanced Instruments – launched the VIPS™ single cell seeding platform. The system accelerates cell line development workflows for CHO, HEK and iPSC-derived cells and has a unique ‘Double-Lock of assurance’ that provides two pieces of evidence of clonality at different stages in the process. The VIPS platform has become an integral part of major cell line development projects around the world. However, the challenge of meeting stringent regulatory requirements for GMP workflows has limited the use of the platform in the development of new iPSC-based cell therapies.
Here, we talk to two experts at Advanced Instruments – Dr. Ian Taylor, Director of Business Development, who has played a pivotal role in the company’s cell therapy strategy, and Dr. Marta Rucka, Global Product Leader for the company’s VIPS and Cell Metric® systems – about how they built on the successes of the VIPS to develop a new platform that is tailored for single-cell cloning of iPSC-derived cells, the VIPS PRO.
Tailored to fit cell therapy workflows
“It wasn't just something we dreamt up and did on our own,” explains Taylor. In 2021, the company teamed up with multiple iPSC therapeutics companies to help them develop a new single-cell seeding platform tailored to their needs. Taylor adds, “the iPSCs experts quickly adopted our VIPS system and provided us with crucial feedback on development of a new platform suitable for GMP workflows.”
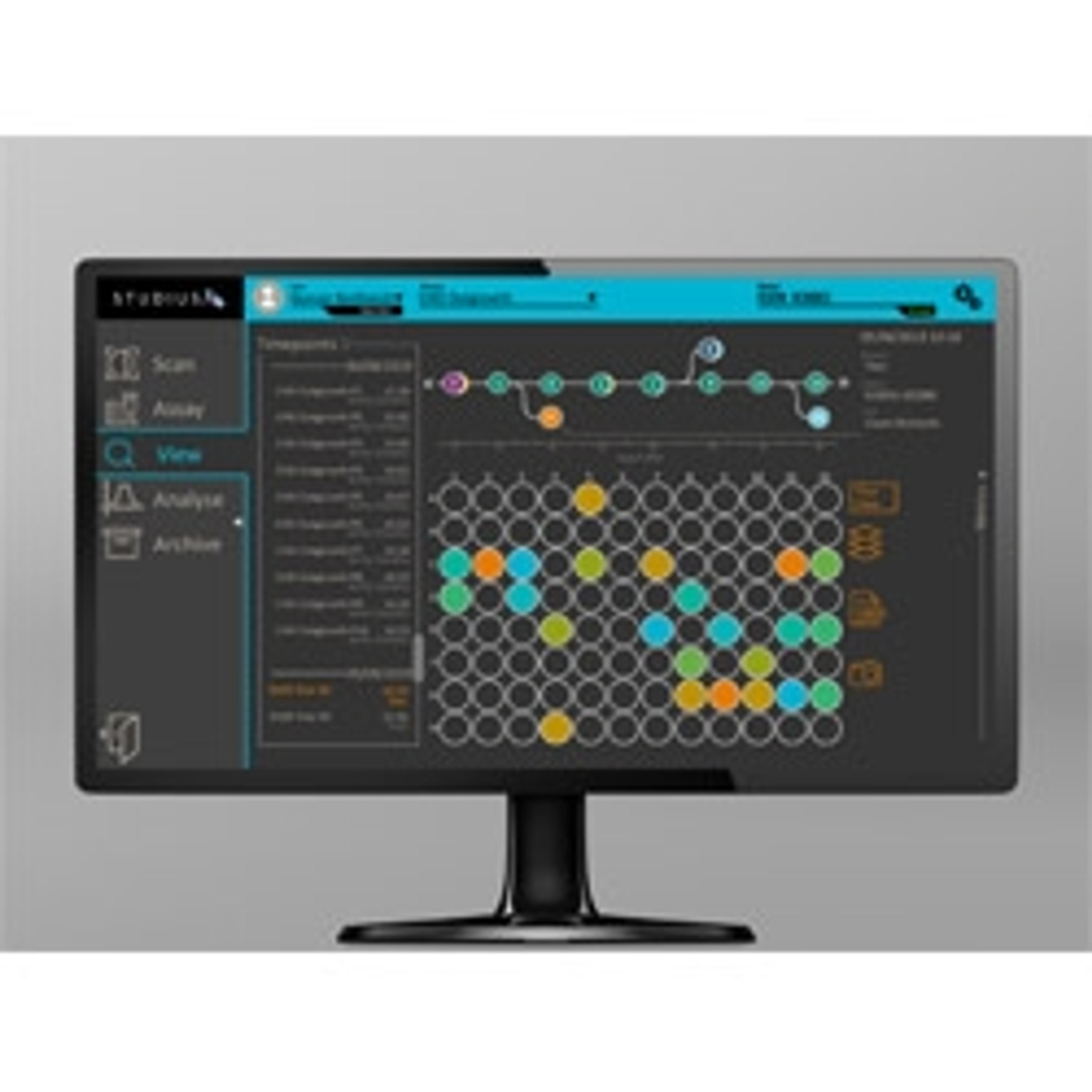
Based on the customer feedback, the Advanced Instruments R&D team worked on developing a next-generation platform for single-cell seeding – VIPS PRO. While keeping the system’s core seeding technology the same, the team introduced a number of changes to improve performance and ease of use, while ensuring GMP-compatibility. Those features included integrating STUDIUS™ software to meet the stringent requirements of FDA 21 CFR part 11 including audit trail, introducing validated single-use seeding kits, and changing the system’s shape to increase the performance in the downflow air of the biological safety cabinet. Plus, intelligent cell detection and ‘Double-Lock assurance’ technologies were incorporated, and a library of standard operating procedures was produced.
Taylor says, “we were making sure we ticked every box with some real therapeutics customers so that we knew the VIPS PRO would be completely fit for purpose for other companies just like them.”

An ideal platform for iPSC therapeutics
The VIPS PRO retains many of the key features of the original system but with improvements to ensure compliance to requirements for GMP manufacturing. Rucka explains, “The VIPS PRO is a perfect solution for customers in GMP because, not only does it give the customer confidence in compliance with data integrity standards, they also get the ‘Double-Lock assurance’ of monoclonality and this is well documented for any IND submissions.”
One key improvement to the STUDIUS software is a new sophisticated AI-driven algorithm to recognize iPSC cells and distinguish them from any cell debris or air bubbles that may be in the well. The Advanced Instruments’ Cell Metric high contrast imager also benefits from the STUDIUS software updates, enabling easy integration of both instruments. Rucka says, “we've always been really focused on providing the best solution for customers and that's why we put such a huge focus on the IT and artificial intelligence and the software development.”
Unlike the original VIPS system, which uses ‘wash and reuse’ cell reservoirs, the VIPS PRO uses single-use seeding kits, which will appeal to customers developing cell lines to manufacture monoclonal antibodies and iPSCs for GMP applications that need to meet the strictest of regulatory requirements. “For that group of customers, having single-use disposable cell reservoirs will be absolutely key to their regulatory submission,” explains Taylor.
Although the VIPS PRO has only just launched, it is already popular with existing VIPS users with many of the early adopters being first in line to purchase the new VIPS PRO as it rolled off the production line. Taylor says, “one customer in Canada has actually designed their GMP facility specifically around the VIPS PRO. That is the effect it’s had on them.”
An exciting future in cell therapy
Advanced Instruments believes that the VIPS PRO and other cell line development solutions have key roles to play in decreasing the cost of and streamlining the process of cell therapy manufacturing. Previously the focus has been on designing cell therapies to treat cancers but some of their customers are now developing treatments for other conditions such as diabetes and heart disease. As the field continues to expand there is a continuous demand for novel solutions. Rucka says, “We really look forward to working with those customers and see what new technologies and new solutions we can provide.”
Learn more about the VIPS PRO >>