Cisbio Launches HTRF<sup>®</sup> Terbium Platform for GPCR Screening at SBS
10 Apr 2008Cisbio, a global developer of HTRF® (Homogeneous Time-Resolved Fluorescence) technology and services used in assay development and drug screening, today announced the launch of its HTRF Terbium platform for GPCR screening, with the introduction of IP-One Tb and cAMP Tb. These assays mark the first products in Cisbio’s second-generation HTRF technology pipeline to incorporate the Lumi4™-Tb cryptate for enhanced screening performance.
Lumi4-Tb, a Terbium cryptate developed by Lumiphore, Inc. and licensed exclusively to Cisbio for the drug discovery field of use, is the newest member of Cisbio’s family of cryptates on which HTRF chemistry is based. HTRF is Cisbio’s highly sensitive, robust technology for the detection of molecular interactions in vitro and is widely used for primary and secondary screening phases of drug development. Lumi4-Tb’s structure, a lanthanide tightly embedded in a surrounding macrocycle, remains very much in line with that of previous HTRF cryptates and shows superior stability compared to other Terbium complexes. It therefore allows for enhanced assay performance in terms of sensitivity, assay window, and robustness, without compromising the features and benefits that HTRF brings to assays: a “mix and measure” non-radioactive format, miniaturizable to uHTS formats, showing excellent robustness and a low compound interference rate.
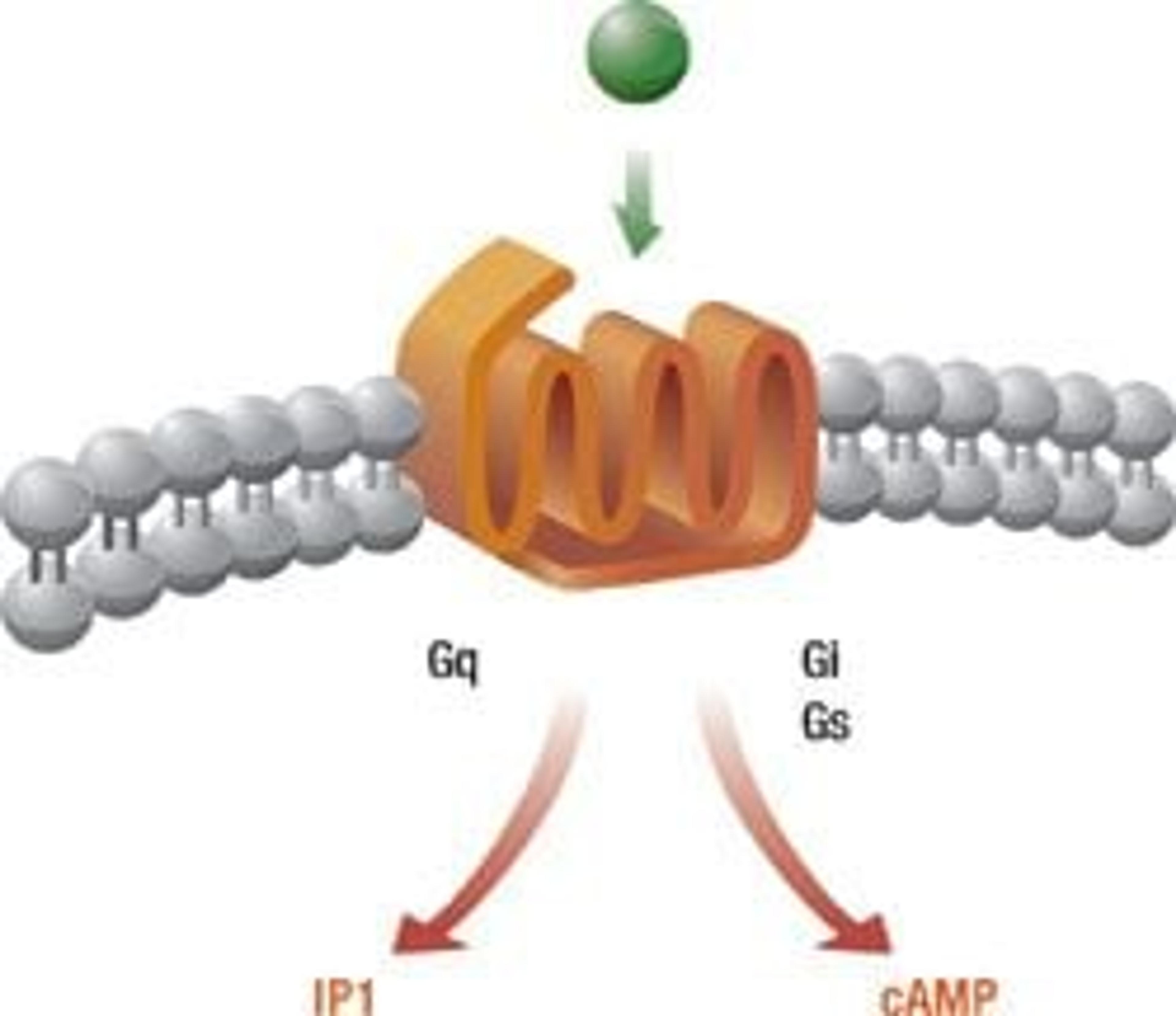
Cisbio has long been a leader in the development of tools for researchers in G-Protein Coupled Receptors (GPCRs), one of the most important target classes investigated within drug discovery. Launched in 2005, IP-One was the first cell-based high throughput system to easily detect inositol(1)phosphate (IP1), one of the major products of the phosphatidyl inositol cascade and which tightly correlates with Gq-coupled activity. Reformatted with Lumi4-Tb instead of a Europium cryptate, IP-One Tb offers increased sensitivity and an enhanced signal-to-noise ratio, and can be easily adopted by current users as it involves the same protocol as before. Cisbio’s cAMP assays, which run on the same industry-standard hardware and follow the same methodology and procedure as IP-One, have also been extended to include cAMP Tb, thereby creating a Terbium technological platform for investigators to assess all GPCR targets that can be used with any existing HTRF-compatible instrument.
“As a pioneer in TR-FRET technologies, Cisbio recognized the need to create a new generation of functional tools to meet a new generation of industry needs,” said François Degorce, head of HTRF Marketing and Business Development at Cisbio. “We selected the best possible chemistry to complement our robust Europium cryptates, and this new Terbium cryptate will enable us to provide GPCR researchers with enhanced, easy-to-use tools that further improve assay implementation.”
The incorporation of Lumi4-Tb into IP-One Tb and cAMP Tb is the first step towards integrating this new chemistry into Cisbio’s broad range of assays. Lumi4-Tb will also be available on request to Cisbio’s customers for custom labelling and assay development services.