Cologuard Plus test outshines fecal immunochemical test in pivotal BLUE-C study
Cologuard Plus was evaluated against an independent fecal immunochemical test, which it significantly outperformed
26 Mar 2024
Exact Sciences Corp., a leading provider of cancer screening and diagnostic tests, has announced the online publication of the BLUE-C study results in The New England Journal of Medicine. The peer-reviewed study, 'Next-Generation Multitarget Stool DNA Test for Colorectal Cancer Screening', also appears in the journal's March 14, 2024 print issue.
The 20,000-participant BLUE-C study was designed to determine the performance characteristics of Exact Sciences’ next-generation multitarget stool DNA test, Cologuard Plus, for colorectal cancer (CRC) and to compare that performance to the fecal immunochemical test (FIT), a commonly used noninvasive CRC screening test. Exact Sciences submitted to the US Food and Drug Administration (FDA) its pre-market approval (PMA) application for Cologuard Plus in December 2023, including complete results from the BLUE-C study, and plans to make the test available in 2025, pending approval.
Cologuard Plus met all BLUE-C study endpoints, demonstrating 94% sensitivity for CRC at 91% specificity including non-advanced findings, and 93% specificity including no findings. Specificity was even better in younger age groups, at 96% in 45–54 year olds. Cologuard Plus will minimize unnecessary follow-up colonoscopies by reducing the likelihood of a false-positive screening test.
Results from BLUE-C also show Cologuard Plus significantly outperformed an independent FIT* for overall CRC sensitivity, treatable-stage CRC (stages I–III) sensitivity, high-grade dysplasia sensitivity, and advanced precancerous lesion sensitivity.
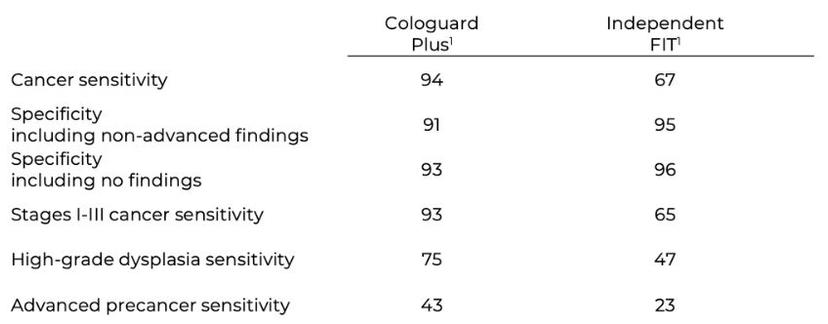
To potentially expand the eligible screening population beyond average-risk patients, the BLUE-C study enrolled a small subset of participants with a first-degree relative with a history of CRC. Sensitivities for CRC and advanced precancerous lesions were similar among participants with a first-degree relative with a history of CRC and those without such a relative.
Among the subset of nearly 19,000 participants who were average-risk, without a first-degree relative with a history of CRC, Cologuard Plus exhibited 95% sensitivity for CRC and 43% sensitivity for advanced precancerous lesions at a 91% specificity including non-advanced findings, or 94% specificity including no findings.
The prospective, multi-center BLUE-C study utilized colonoscopy as a reference method, directly comparing Cologuard Plus and an independent FIT. BLUE-C investigators also collected blood samples for evaluation of a blood-based CRC screening test developed by Exact Sciences.
The BLUE-C study cohort was diverse and reflective of the US population. About 40% of all participants identified as Hispanic or Latino, Black, Asian, American Indian or Alaska Native, or Pacific Islander. This enrollment diversity helps ensure that the BLUE-C findings and Cologuard Plus are relevant for all screen-eligible individuals, regardless of race or ethnicity.
*The commercially-available Polymedco OC-Auto® Micro 80 iFOB Test.
References
Imperiale T.F. et al. Next-Generation Multitarget Stool DNA Test for Colorectal Cancer Screening. N Engl J Med. 2024 Mar 14; 390(11):984–993.
