Cut Through the Haze: Can the Latest 3D Imaging Technology Help You Remove Out-of-Focus Blurring?
In this article, discover how Computational Clearing technology from Leica Microsystems quickly resolves the longstanding challenge of blurry contrast when imaging thick sections
17 Jun 2019
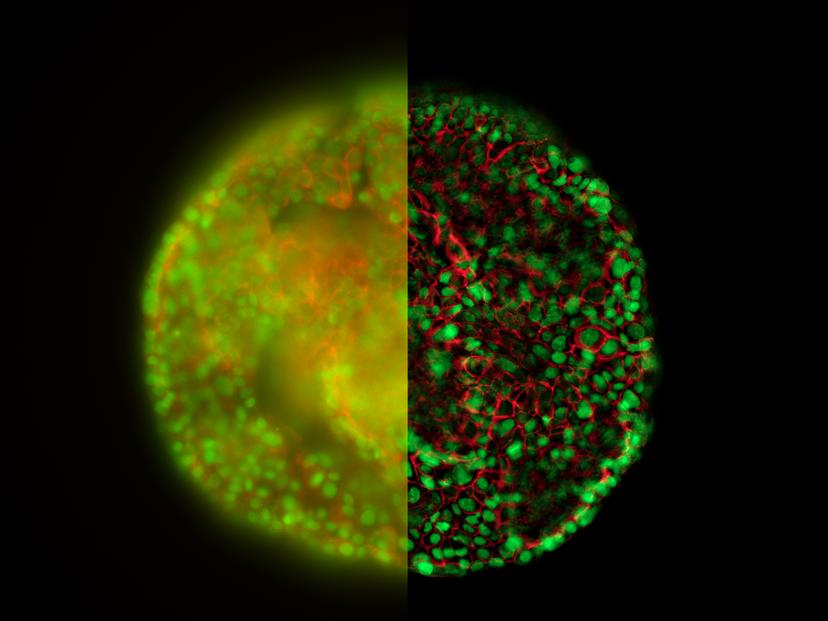
When placing a 3D specimen under a fluorescence microscope, every scientist faces a familiar challenge: a blurry image. This typical haze, resulting from out-of-focus regions of the specimen, blocks off the structural details required for further analysis.
Although there is an increasing need for 3D imaging to answer big biological questions, the traditional solutions to circumvent out-of-focus haze involves cumbersome, time-consuming, post-processing methods.
A unique technology introduced by Leica Microsystems resolves the out-of-focus haze problem in real time. In this interview, we speak to Market Development Manager, Dr. André Quinkertz, about Leica’s Computational Clearing technology in its latest THUNDER Imagers that enable scientists to “see through the haze”.
“People are not able to use these hazy images unless they run them through a lengthy deconvolution process, which is often another software package and takes a long time,” says Quinkertz. “The time required to make sure the haze is removed is a big hurdle. And this is exactly the reason why we developed the THUNDER Imagers — to get rid of this pain of waiting.”
Sharper contrast with Computational Clearing technology
The new opto-digital Computational Clearing technology on Leica’s THUNDER Imagers detects and removes out-of-focus blur for images in real time. The typical haze seen in widefield fluorescence microscope is a result of the combination of the out-of-focus background signals and the desired in-focus signals. The Computational Clearing technology uses an iterative algorithm to clear the out-of-focus haze while keeping the in-focus signals intact. It doesn’t “generate” the desired image, but simply unmasks it by eliminating the unwanted background signals in the sample.
How does the Computational Clearing technology work? Download this application note to learn more about the iterative algorithm used to clear the haze >>
“This means you do not require any lasers, any special hardware in the light path, any confocal parts to do the job and clear the images,” adds Quinkertz. “The THUNDER Imagers allow the user to see clearly, even deep within a sample and to get first-rate results very fast.”
Reliable, reproducible and unbiased data
When working with traditional imagers requiring tedious post-processing steps, a common human error is to ‘cherry pick’ the best results as the final images don’t represent all the data points. One of Leica’s customers working on cancer tissue asked, ‘How do I get rid of people unintentionally picking biased images that look nice but leave out the ones that also contribute to the statistics?’. The THUNDER workflow enables 100% coverage of the tumor section without having to wait long, thereby eliminating any unintentional cherry picking of results. “This is how the system supports the workflow to give you a better database for your science. It is so fast and easy to set up that it doesn't conflict with the timing of an experiment” says Quinkertz.
The THUNDER imagers are integrated with fast-moving stages, LED-based light and fully controlled hardware and software to help deliver a simpler user interface. The scientist’s time and energy can be focused on the scientific problem and not the image processing itself. “The goal must always be to get better data points, better statistics, more reliable results,” says Quinkertz.
Jennifer Kulhei, Leica Microsystems, explains the challenges posed by hazy images in widefield microscopy and how the Computational Clearing technology can help resolve it.
Maintaining cellular physiological conditions
To get optimal results from 3D cell culture, it’s important to maintain the imaging conditions as close as possible to physiological conditions. “3D cells are used as a model to study tumor development or the reaction to new pharmacological substances. It implies that everything you do should be as close as possible to natural conditions” says Quinkertz.
To minimize photobleaching, the THUNDER Imagers use low light intensities and short exposure times, as well as limiting illumination to the actual recording time.
“The longer the sample stays on the microscope stage, the more the risk of physiological conditions changing and data quality decreasing. This is where the hardware features really support the workflow,” says Quinkertz. “It offers a fast and reliable stage to get the job done quickly so you can compare the data points across multiple wells.”
Plus, the incubator in the THUNDER Imager 3D Live Cell & 3D Cell Culture helps maintain the humidity, temperature and CO2 levels needed to keep the cells as close to their natural state as possible.
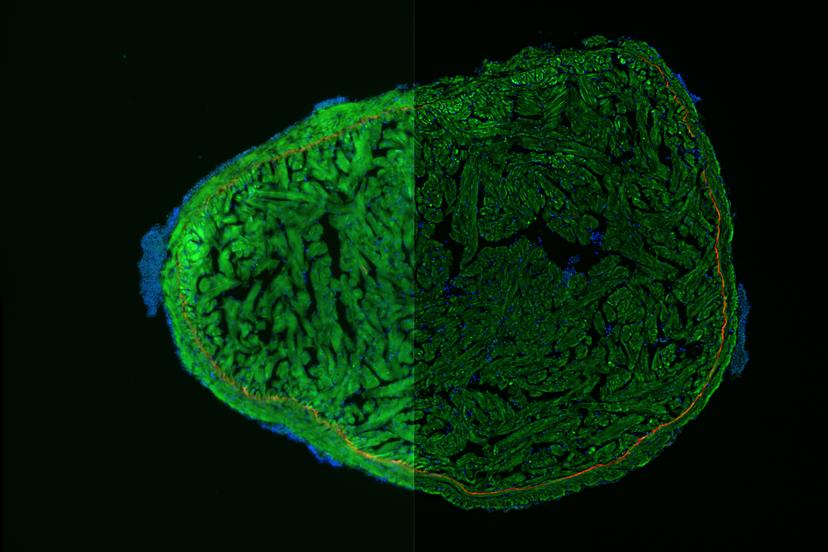
When working with live cells or 3D spheroids, time is an important factor. The THUNDER Imager stage moves at the rapid rate of 10 positions per second to enable time-lapse imaging. “It can fundamentally change the way that people work when imaging model organisms, tissue sections and 3D cell cultures that require fast imaging,” states Quinkertz.
Making 3D analyses easier and faster
Achieving good quality, haze-free images in thick sections was only formerly possible with confocal microscopes. “We see that 3D model analysis has become more and more complex. There's more downstream analysis and big data. So, people analyze a lot of images” explains Quinkertz. “For us, the first question was, how can we make sure that people get good images fast and easily for downstream analysis?”
“THUNDER Imagers are unique because the Computational Clearing technology is not offered by anyone else,” notes Quinkertz. “It solves a very old and annoying problem – how to use widefield imaging in the best way and how to get rid of this haze.”
Adaptive 3D imaging of cells, organisms or tissue sections
The THUNDER Imager family can be used for three different types of applications:
- 3D live cells
- Model organisms
- 3D tissues

Equipped with the same Computational Clearing technology, the three systems differ in external components specific to particular applications. “For example, the model organism imager has a large field of view and working distance so you can place a petri dish directly underneath it and look at it from the top,” explains Quinkertz.
With such adaptive technology, the THUNDER imagers are not only designed to set new benchmarks for performance but will also grow with your future needs – making now the right time to tackle biologically-relevant 3D models.
Visit our 3D Cell Culture Special Feature for more on the latest advances in the field>>