Detection of intrathecal antibodies in CNS diseases
Euroimmun offers a range of CE-marked ELISAs for determination of intrathecal antibodies in cerebrospinal fluid samples
14 Sept 2023
The investigation of antibodies against pathogenic agents in cerebrospinal fluid (CSF) samples is an important tool in the diagnosis of acute or chronic inflammation of the central nervous system (CNS). Euroimmun offers a range of CE-marked ELISAs for the determination of intrathecal antibodies in CSF and serum pairs, complemented by software for fully automated evaluation of results.
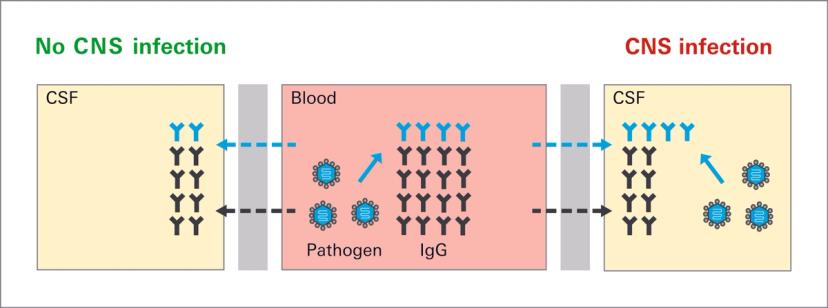
Acute CNS infections
Acute infections of the CNS can be caused by a range of viruses, bacteria, protozoa, and fungi. Although rare, they can present as life-threatening emergencies, requiring rapid diagnosis. Clinical manifestations include meningitis, meningoencephalitis, or encephalitis. Symptoms can be unspecific, for example, fever, headache, and alternated mental state. Identification of the causative agent is essential for providing appropriate treatment. Diagnosis typically involves a combination of physical examination, blood tests, imaging studies, and analysis of CSF obtained by lumbar puncture (spinal tap)1.
CSF analysis
Detection of specific antibodies in CSF supports the diagnosis of CNS infections. It is especially helpful in situations where direct methods such as PCR have a low positivity rate, for example, due to rapid pathogen clearance or in chronic stages of infection. The analysis is recommended by the German Society of Neurology (DGN) as part of the diagnostic work-up for suspected CNS infections. It is used alongside other CSF laboratory tests such as cell count, total protein, lactate, oligoclonal bands, cytology, and direct pathogen detection.
In the DGN diagnostic guidelines for Lyme neuroborreliosis and neurosyphilis, in particular, detection of intrathecal antibodies against the respective causative organism (Borrelia or Treponema pallidum) serves as a main criterion for confirming a diagnosis2, 3.
Chronic inflammation in MS
Some non-infectious chronic inflammatory diseases such as multiple sclerosis (MS) trigger a polyspecific humoral immune response, leading to intrathecal production of antibodies against various non-causative agents. The detection of intrathecal antibodies against measles virus, rubella virus, and/or varicella zoster virus (VZV) is a specific indicator of MS and can help to differentiate MS from clinically similar diseases. Machine readable zone (MRZ) testing in patients with suspected MS is recommended by the German Society for Cerebrospinal Fluid Diagnostics and Clinical Neurochemistry in its current guidelines4.
Antibody index
In CNS infection diagnostics, it is important to distinguish intrathecally produced antibodies from antibodies that have diffused from the blood into the CSF. This is done by setting the concentration of specific antibodies in CSF and serum in relation to total immunoglobulin. The calculated quotient is known as the antibody index (AI). An AI value of more than 1.5 indicates pathogen-specific antibody production in the CSF. In cases of elevated total IgG in CSF, as occurs with polyclonal antibody stimulation in MS, the quotient of albumin in CSF to serum is additionally measured. This is used to derive the limes quotient, which is used instead of the total IgG quotient in the calculation of the AI5.
ELISAs for CSF/serum pairs
Specific antibodies in CSF and serum pairs can be measured quantitatively using specialized ELISAs. A broad range of CE-marked CSF ELISAs for the detection of intrathecal antibodies is available from Euroimmun encompassing the parameters herpes simplex virus 1 and 2 (HSV-1/2), chronic active Epstein-Barr virus (EBV-CA), VZV, measles virus, mumps virus, rubella virus, tick-borne encephalitis (TBE) virus, cytomegalovirus (CMV), Borrelia, Treponema pallidum and Toxoplasma gondii.
The processing of the ELISAs is standardized with identical incubation schemes and exchangeable reagents. This enables easy parallel analysis of different parameters, as well as efficient automation. Results are quantified by means of 4- to 6-point calibration curves or using a single recalibrator with reference to a stored master curve generated by EUROLabCSF software. The use of a single calibrator increases the cost-effectiveness since fewer ELISA wells are required per analysis.
Automated AI calculation
To facilitate the result evaluation, Euroimmun has developed EUROLabSCF software, which automatically calculates the quotients for pathogen-specific antibodies, total immunoglobulin, and albumin, as well as the limes quotient and AI. The results are displayed clearly in quotient diagrams according to Reiber and Lange. The software communicates bidirectionally with the processing instruments and the LIS so that time-consuming and error-prone manual data transfer is no longer required. The CSF antibody analysis is thus fully automatable from start to finish, providing a high level of efficiency and standardization.
Quality assessment
External quality assessment institutes such as INSTAND and ESfEQA offer programs for CSF diagnostics to help diagnostic laboratories to meet the high analytical standards required. These schemes cover a range of parameters such as measles virus, rubella virus, VZV, HSV-1/2, TBEV, and Borrelia. In the INSTAND CSF schemes from October 2022, results obtained using Euroimmun CSF ELISAs matched the target values in 92% to 99% of the samples. This represented the highest pass rate of all tests used.
CXCL13 in neuroborreliosis
The chemokine CXCL13 is an additional CSF marker that can aid early identification of neuroborreliosis. High concentrations of CXCL13 in CSF are often detected before antibodies appear. CXCL13 determination can thus help close the diagnostic gap between infection and positive antibody test and identify neuroborreliosis at an earlier stage. CXCL13 is, moreover, useful for detecting reinfections, where intrathecal antibodies may be present from previous infection. CXCL13 also serves as a marker for therapy monitoring, as its concentration in the CSF sinks rapidly with successful treatment. CXCL13 can be determined by ELISA. Euroimmun offers the first CE-marked ELISA for this application.
Summary
- Euroimmun offers a comprehensive range of CE-marked assays for the detection of intrathecal antibodies in CSF samples
- Results are quantified using a 4-6-point standard curve or a single recalibrator with reference to a stored master curve
- Euroimmun also offers the first CE-marked ELISA for measurement of CXCL13 in CSF
- Complete automation solutions are available for standardized processing and result evaluation
References
Tumani H. et al. S1 guidelines “lumbar puncture and cerebrospinal fluid analysis” (abridged and translated version) Neurological Research and Practice 2020; 2:8. https://doi.org/10.1186/s42466-020-0051-z
Rauer et al. Clinical practice guideline Lyme neuroborreliosis. Dtsch Arztebl Int 2018; 115: 751–6. doi: 10.3238/arztebl.2018.0751
Klein M. et al. German guidelines on the diagnosis and treatment of neurosyphilis. Neurol Res Pract 2020 Nov 17;2:33. doi: 10.1186/s42466-020-00081-1
Jarius S. et al. The MRZ reaction as a highly specific marker of multiples sclerosis: re-evaluation and structured review of the literature. J Neurol 2017; 264(3): 453-466. doi: 10.1007/s00415-016-8360-4
Reiber H. Knowledge-base for interpretation of cerebrospinal fluid data patterns. Essentials in neurology and psychiatry. 2016; Arq. Neuro-Psiquiatr. 74 (6). doi: 10.1590/0004-282X20160066
