Development of a Luminex xMAP® Assay to Detect Amyloid β Oligomers in Alzheimer’s Disease Cerebrospinal Fluid
written by Luminex
5 Jan 2016

written by Luminex
Alzheimer’s Disease (AD) is one of the most common forms of dementia. AD affects more than 27 million Americans—most of whom are over 85 years old. For decades, researchers couldn’t quite understand the onset or progression of this disease—but now, they have found that certain pathological hallmarks lend clues into how the disease progresses and how it can be treated. Of these hallmarks, amyloid beta (Aβ), neurofibrillary tangles, and neuritic plaques are of the most importance, especially pertaining to determining a neuropathologic diagnosis. Cerebrospinal fluid is also of interest to researchers analyzing biochemical and neuropathogic changes in AD patients.
Although there are many published studies highlighting the fact that soluble amyloid beta aggregates have a toxic effect on the brain (disrupting memory and synaptic plasticity), there is conflicting evidence about whether the levels of amyloid beta oligomers rise during AD pathogenesis.
Reason for Evaluation
According to established data, the measurement of amyloid beta 42 (Aβ42), total tau, and phosphorylated tau is essential in diagnosing Alzheimer’s Disease. Previous research has shown that all three analytes should be tested in order to provide the most accurate diagnosis (when compared to identification of a single protein marker) for up to 90% accuracy,1 although a recent study2 has shown that measurement of Aβ1-42 and p-Tau levels in the CSF is sufficient to diagnose AD. Traditionally, ELISA has been used to test each analyte, and results are then compared to create an accurate diagnosis. However, for this study, researchers part of the Alzheimer’s Disease Neuroimaging Initiative compared the Alzbio3 assay run on the Luminex platform to analyze all three markers in the same sample, as well as a custom assay detecting Aβ oligomers they developed on Luminex xMAP Technology. The goal was to determine if oligomeric Aβ levels correlated with the other AD biomarkers.
Method of Testing and Participants
For this study, researchers selected 20 Alzheimer’s patients, which they age matched with 19 controls. All participants underwent neuropsychological evaluations and the Mini-Mental State examination to determine eligibility. Lumbar punctures were performed to obtain CSF for the study.
These samples were used to detect and measure aβ42, total tau, and phosphorylated tau levels using a commercially available AlzBio3 kit. This kit was capable of testing all three levels in one platform. The researchers also tested the same samples using the custom assay to detect oligomeric aβ levels.
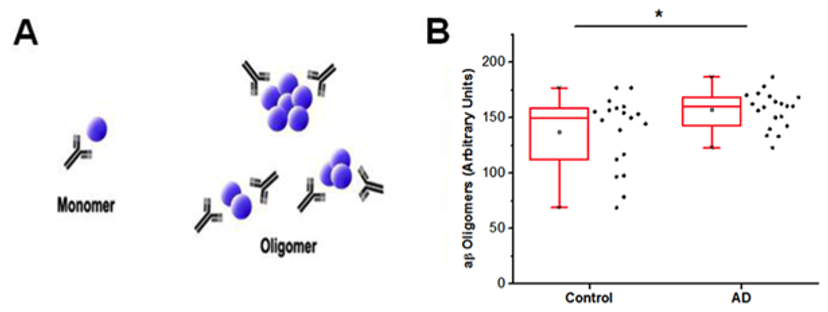
A: Schematic of SAS design illustrating the use of the same monoclonal antibody for both capture and detection with aβ42 represented by blue spheres. Monomeric species are not able to bind both capture and detect antibody; however, higher order aggregates will have two or more epitopes for Ban50 binding and signal generation. B: Using the SAS design with Ban50 antibody, AD cases have more oligomeric aβ42 relative to age and gender matched control CSF (Mann Whitney U = 114, p = 0.03, two-tailed). * indicates difference between groups is statistically significant with p < 0.05.1
Results
Using the Luminex platform, researchers were able to detect elevated aβ oligomers in the AD patients compared to control subjects (p < 0.05). In addition, an elevated ratio of aβ to aβ4 was detected in AD patients when compared to the controls. Additional research will be needed to determine whether analysis of oligermic Aβ could possibly be used as an evaluation method for late onset AD patients.2
The original article was posted by Luminex here.
References
1. Herskovitz A, Locascio J, Peskind E, Li G, Hyman B. A Luminex assay detects amyloid β oligomers in Alzheimer’s Disease cerebrospinal fluid. PLoS ONE 2013;8(7):e67898. doi:10.1371/journal.pone.0067898.
2. Bombois S, Duhamel A, Salleron J, Deramecourt V, Mackowiak MA, Deken V, Sergeant N, Pasquier F, Buée L, Sablonniére B, Schraen-Maschke S. Curr Alzheimers Res 2013;10:357-364. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23061918.