Droplet microfluidics for single cell analysis: The next generation of capability
Discover how droplet microfluidics is revolutionizing single-cell analysis and selection in biopharma
9 Sept 2024Droplet microfluidics is an established technology for single-cell analysis, characterization, and selection. Cells are encapsulated in droplets of a suitable aqueous media contained in an oil carrier fluid, and the droplets are stabilized with a surfactant. The droplets are produced using microfluidic methods, where liquids are pumped through carefully designed geometric channels at the micrometer scale (Figure 1). By varying the relative flow rates of the cell suspension and oil carrier fluid, the distribution of the number of cells per droplet can be controlled, particularly to obtain predominantly single cells per droplet based on the Poisson statistical distribution. These droplets can then be subjected to different operations, all within the closed microfluidic system.
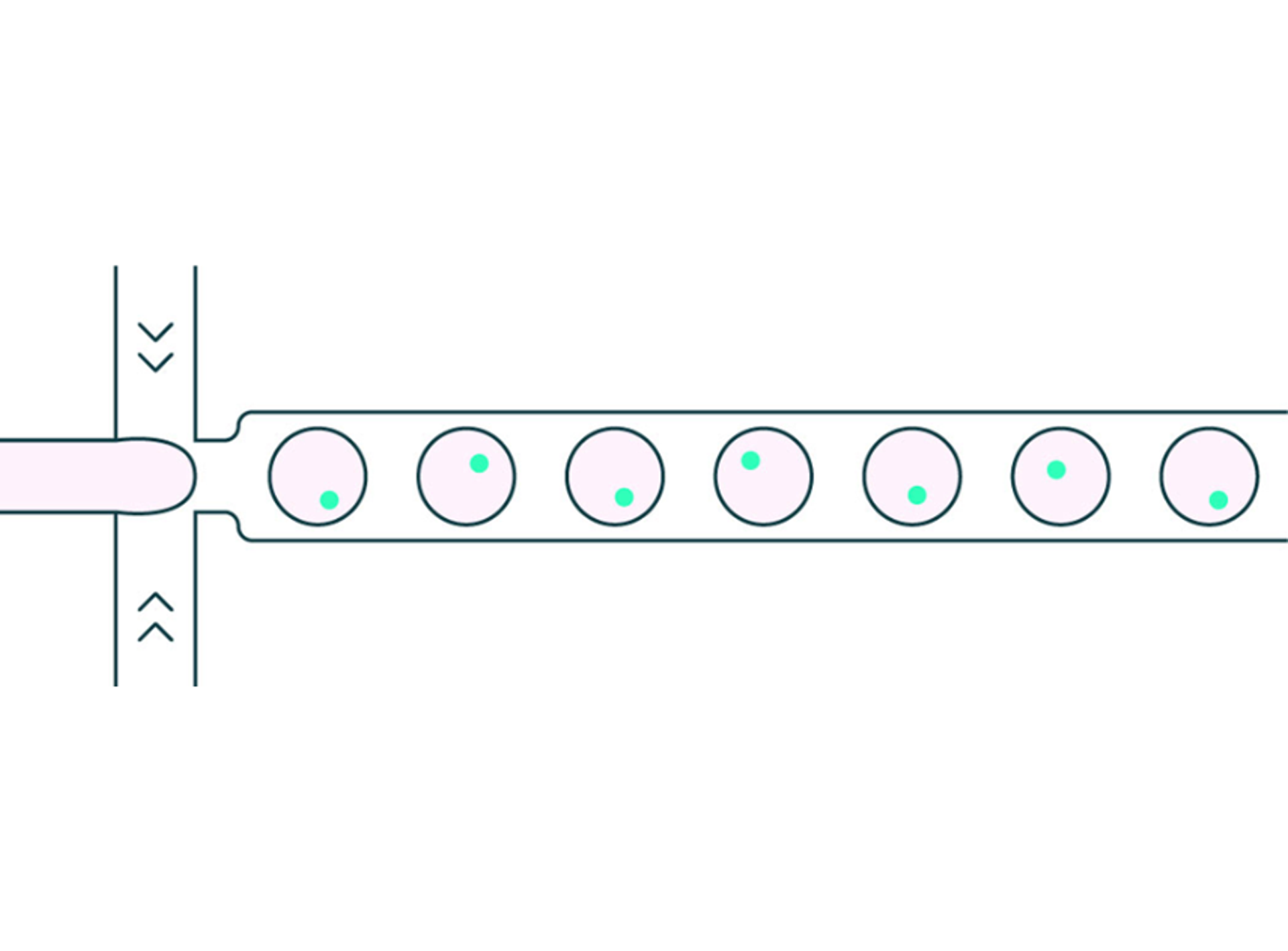
Figure 1: droplet generation process to encapsulate a single cell per droplet
The adoption of this technique within biopharma is driven by the real-world benefits it brings.
- Localization: Individual droplets form self-contained reaction vessels which allow secreted molecules to be analyzed as well as surface markers. The very small droplet volumes allow rapid increase in concentration of the target to support robust assay performance.
- Benign environment: Cells are encapsulated in their preferred medium and the oil phase supports gas exchange, and the droplet shields the cell from the shear forces imparted by the microfluidics. Thus, droplets are particularly suited to applications where the cell must maintain viability post assessment.
- Speed and scale: Droplets can be created and processed at high speed, typically into the kHz range. This allows every member of a large cell population to be assessed in a few hours.
- Automation: The flow of droplets through microfluidic channels provides a robust and adaptable platform to automate complex workflows—particularly for selecting cells—and minimize user workload.
- Cost: The small size of the droplets requires minimal amounts of reagent so even large-scale experiments can be performed cost effectively.
A key use case of the technology is cell selection. Here, the need is to start with a large viable heterogenous cell population and find the much smaller subset of cells with certain desired phenotypes while retaining viability (Figure 2).
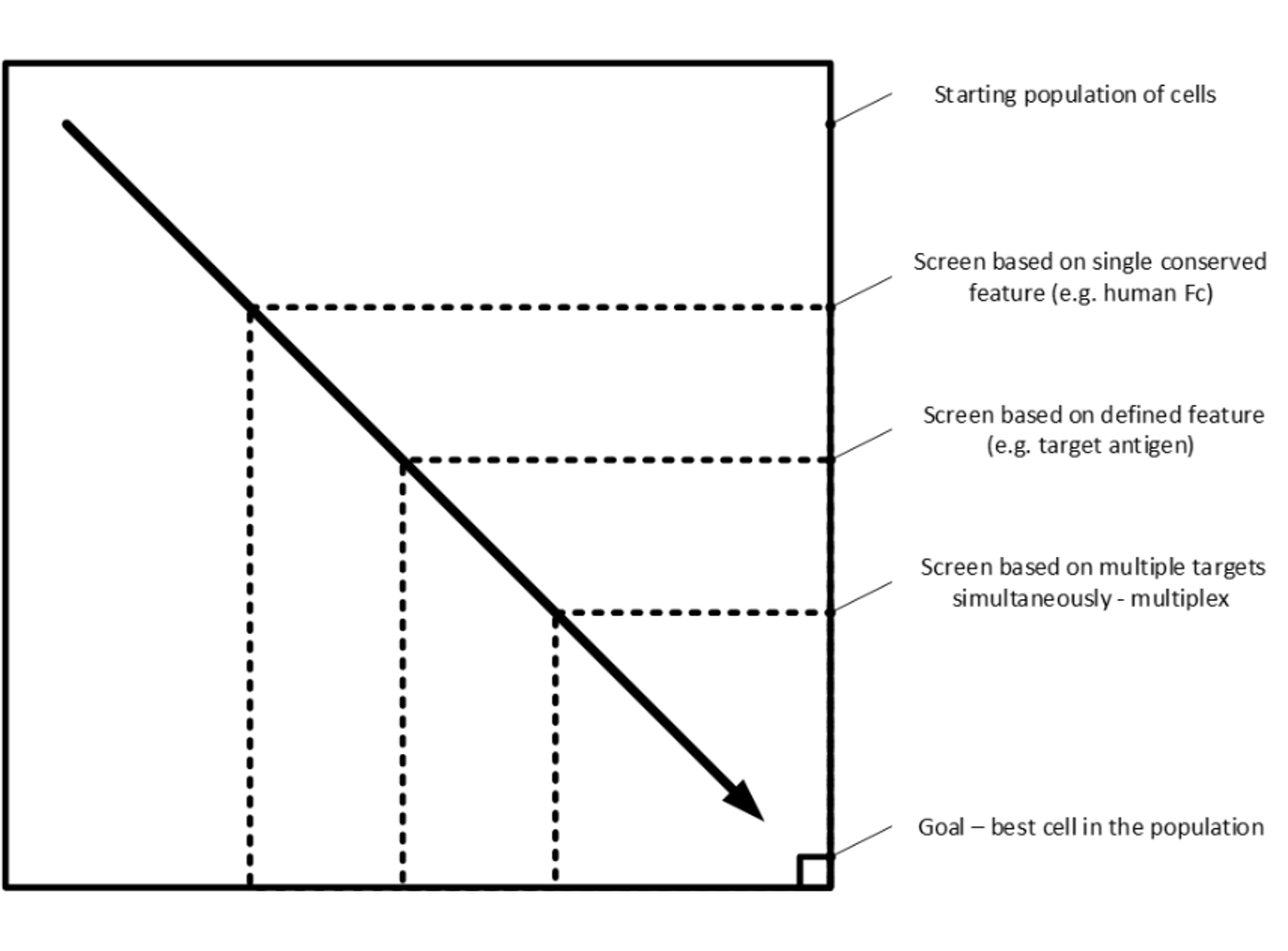
Figure 2: the cell selection challenge – going from the general to the specific as efficiently as possible. Dotted lines represent selection criteria, the better the criteria the closer the boundary to the bottom right-hand corner.
The challenge is to find the most efficient way to move from the general to the specific. Typically, today this process is done in multiple steps using different equipment with the concomitant time and effort needed to run long multi-step workflows. However, droplets provide an approach that can move further and further towards the ideal: doing the whole process in one step from millions of cells to single digits.
The dominant technique used today for the assessment of cells in droplets is fluorescence. This builds on the years of experience in flow cytometry where fluorophores provide a robust signal that can be converted into an electrical signal for logging and processing; and in a format that can be integrated into a bioassay.
Delivering this ideal requires improvements in two areas:
- Asking more precise questions. For example, instead of screening cells for presence or absence of a generic, conserved, phenotype (such as the Fc region of an antibody), screen for the presence of absence of the precise target of interest. Another example would be quantification, moving from a basic presence / absence test to a quantified measure to identify and select only high-producing cells.
- Asking multiple questions at the same time. For example, screen for several markers simultaneously and only accept cells displaying all the markers.
The next generation of droplet-based platforms are tackling these improvements. It requires improvements in all aspects of the technology but particularly assay and detection technologies.
Antigen-specific techniques require new assay approaches, where the user can integrate the desired antigen into a standardized assay quickly and easily for screening. New labelling kits are needed, optimized for the unique properties of the droplet format.
Beyond more specific assays, providing robust quantification in droplets is also technically difficult. The entire measurement system is complex—cell, assay, droplet, consumable, instrument—and the first challenge is providing the underlying precision to provide a repeatable measurement. Delivering this requires great attention to detail in design, component selection, and manufacture. The second challenge is then calibration, referencing back all measurements made to defined standards. This will require innovation in the use of fluor standards in the context of droplet-based systems and how this is integrated into the assay format.
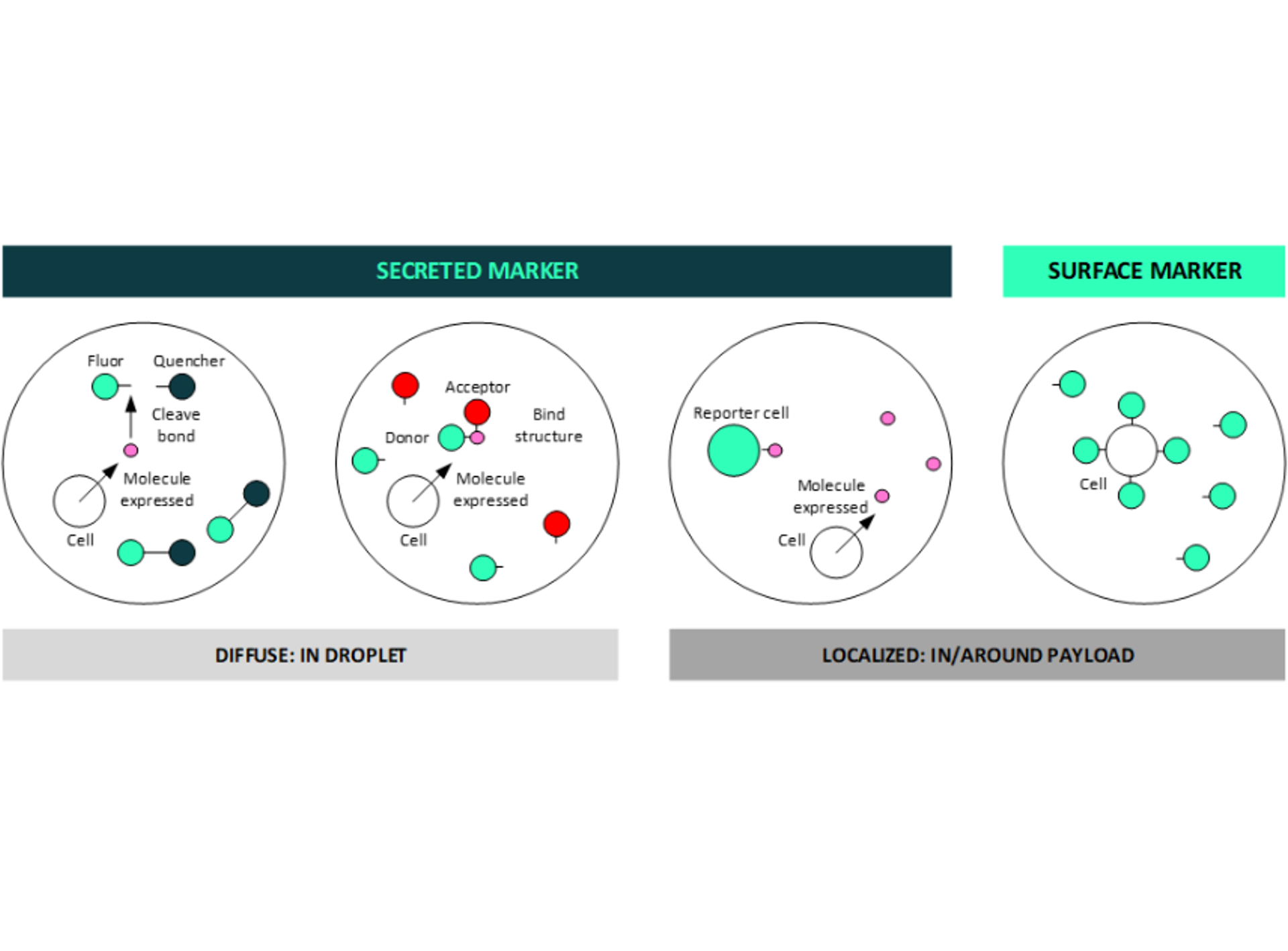
Figure 3: droplets can support both secreted and surface marker detection, a key benefit for deep multiplexing
Droplets are uniquely suited to addressing the multiplex challenge as they can support both secreted and surface marker identification (Figure 3). The secreted molecules can be used either to cleave fluorophore / quencher structures or to bind donor / acceptor pairs in a Förster Resonant Energy Transfer (FRET) type approach thus providing a varying fluorescent signal as the marker is expressed. Alternatively, secreted molecules can be used to generate a fluorescence response, for example using a reporter cell. Conversely, surface markers can be labelled in the more traditional way to generate a localized fluorescent response within the droplet.
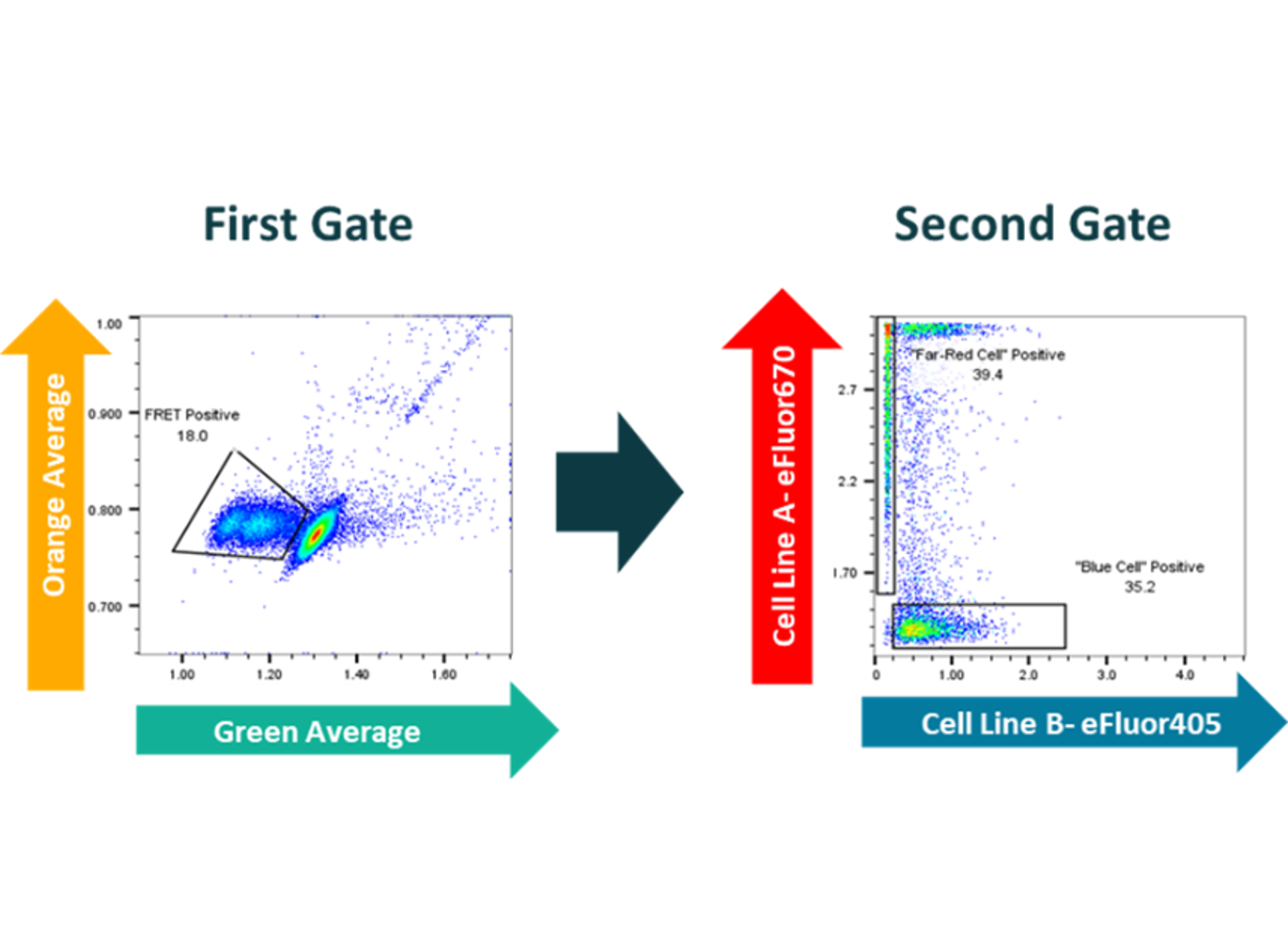
Figure 4: fluorescent multiplexing in droplets assessing both FRET response (gate 1) and cell stain (gate 2) simultaneously
As an example of the power of multiplexing Figure 4 shows data assessing a diffuse marker (a FRET assay) and a localized marker (a cell staining dye) simultaneously. Using gate 1, the population of cells showing FRET response can be selected; and at the same time the two cell types (stained red and blue) can be identified.
Droplet-based systems will continue to play a key part in cell selection workflows as these increasingly sophisticated approaches are developed and brought to market. Novel assays, accurate quantification and deep multiplexing will combine to provide the next generation of capability to identify and select the most important viable cells from increasingly large starting populations quickly and efficiently. Going from millions of cells to single digit numbers is getting closer.
Want the latest science news straight to your inbox? Become a SelectScience member for free today>>

