Environmental Cation Analysis: Essential Application Notes & Information
Find application notes and information resources on modern cation analysis
8 Dec 2016

Image: Shutterstock
Editorial Review by Lois Manton O’Byrne, Editor, SelectScience®
Cations in group I and II of the periodic table are routinely monitored in public water systems in the US, and several other countries. Sodium is monitored by the Safe Drinking Water Act; calcium and magnesium are measured for evaluating water hardness; ammonium ions are monitored in the EU and Japan as they pose a threat to aquatic life.
Analysis methods
Alkali and alkaline earth cations are commonly determined by spectroscopic techniques, as described in EPA 200.5, 200.7, 200.8 and 200.9. However, ammonium cation cannot be measured using atomic absorption techniques and sprectroscopic techniques.
Ion chromatography (IC) is often used to separate earth metals, including ammonium cation in a single analytical run.
Ion chromatography with suppressed conductivity detection has proven to be a robust and reliable technique for the determination of cations. This white paperdiscusses the advantages and disadvantages of suppression in cation-exchange chromatography.
Cations in drinking water, environmental water and waste water
Dissolved alkaline and alkaline earth cations as well as ammonium cations are often analyzed in drinking water, environmental and municipal wastewaters. This analysis is necessary as part of the water monitoring for acceptable taste. Cation analysis in municipal wastewater ensures that there are no environmental effects resulting from the discharge of high-salt concentrations into the water system. Learn more about cost-effective determinations of inorganic cations in municipal wastewater using high-pressure capillary IC in this application note.
Analysis of ammonium cation is important due to its toxicity. Analysis can be carried out using ion chromatography, as described in this technical note, Fast Determinations of Inorganic Cations in Municipal Wastewater.
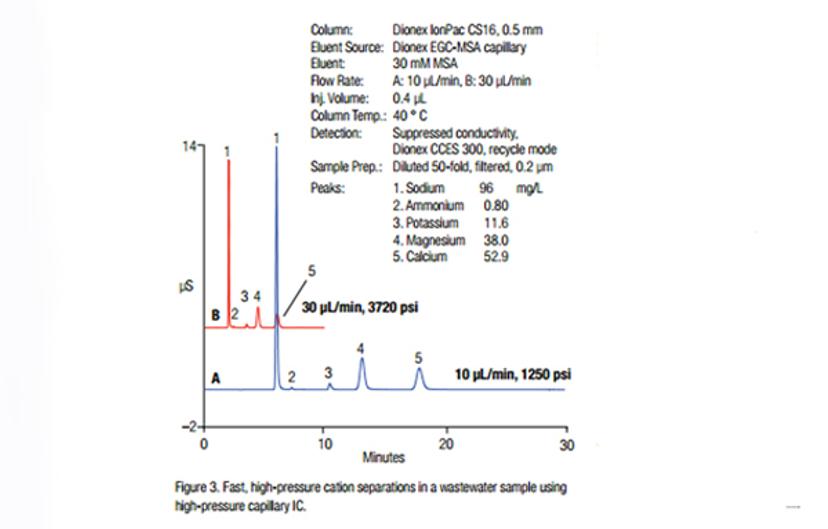
Figure 1: Fast, high-pressure cation separations in a wastewater sample using high-pressure capillary IC1
Ammonium cation can also be analyzed using a discrete analyzer. You can learn more about the determination of water pollutants using photometric analysis in this application note.
Cations in industrial wastewater
Cation analysis in fracking water is used to enhance the recovery of natural gas and oil. As large amounts of water are needed for the fracking procedure, it is becoming common practice to recycle water produced from previous fracking activities. Because cations in the water contribute to scaling problems in water pumps and pipes, their determination in the recycled water is required. This application note describes the determination of cations in hydraulic fracturing flowback water using the Thermo Scientific™ Dionex™ ICS-5000+ Reagent-Free™ High-Pressure Ion Chromatography (HPIC™) system with the Thermo Scientific™ Dionex™ IonPac™ CS16 standard bore (5 mm i.d.) and capillary (0.5 mm i.d.) columns.
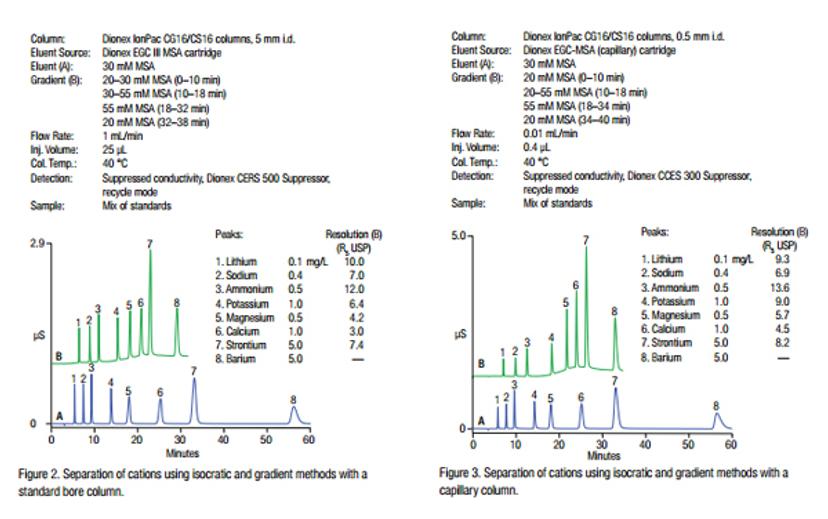
Figure 2: Separation of cations using isocratic and gradient methods with a standard bore column and a capillary column2
Analysis of cation metal contaminants are monitored in power plants as they can accumulate in steam generators or turbines. This buildup can lead to corrosion problems that could lead to serious component failures and plant shutdowns. Read this application note on determination of trace cations in power plant waters, to learn more about a method using the Thermo ScientificDionexIonPacCS14 column to quantify trace concentrations of lithium, sodium, potassium, magnesium, and calcium in the presence of high levels of ammonium and morpholine.
Alkanolamine analysis is important in metal surface finishing and wastewater effluents, as well as in the chemical and pharmaceutical industries for production of emulsifying agents and the manufacturing of laundry additives and dyes. Read this application note to find out more about analysis of alkanolamines using a Thermo Scientific Dionex Series 4500i* with a Pulsed Amperometric Detector (PAD II) or a Pulsed Electrochemical Detector (PED).
Visit Thermo Fisher Scientific’s Cation Analysis Page for more application notes, product selection guides, case studies and more.
References:
1. Application Note: Fast Determinations of Inorganic Cations in Municipal Wastewater
2. Application Note: Determination of Cations in Hydraulic Fracturing Flowback Water from the Marcellus Shale
