Fluorescent DNA Dyes from Biostatus Limited Helping Advance Research in Pancreatic Cell Physiology and Bone Marrow Analysis
1 Jun 2014
Several recent journal papers have demonstrated the success of Biostatus Limited’s fluorescent DNA dyes in a number or research areas; investigating endocrine and exocrine cell physiology, megakaryocyte analysis in bone marrow, and lysis-free analysis of whole bone marrow.
Long-Term, Real-Time Viability Monitoring in ex vivo Tissue Slices
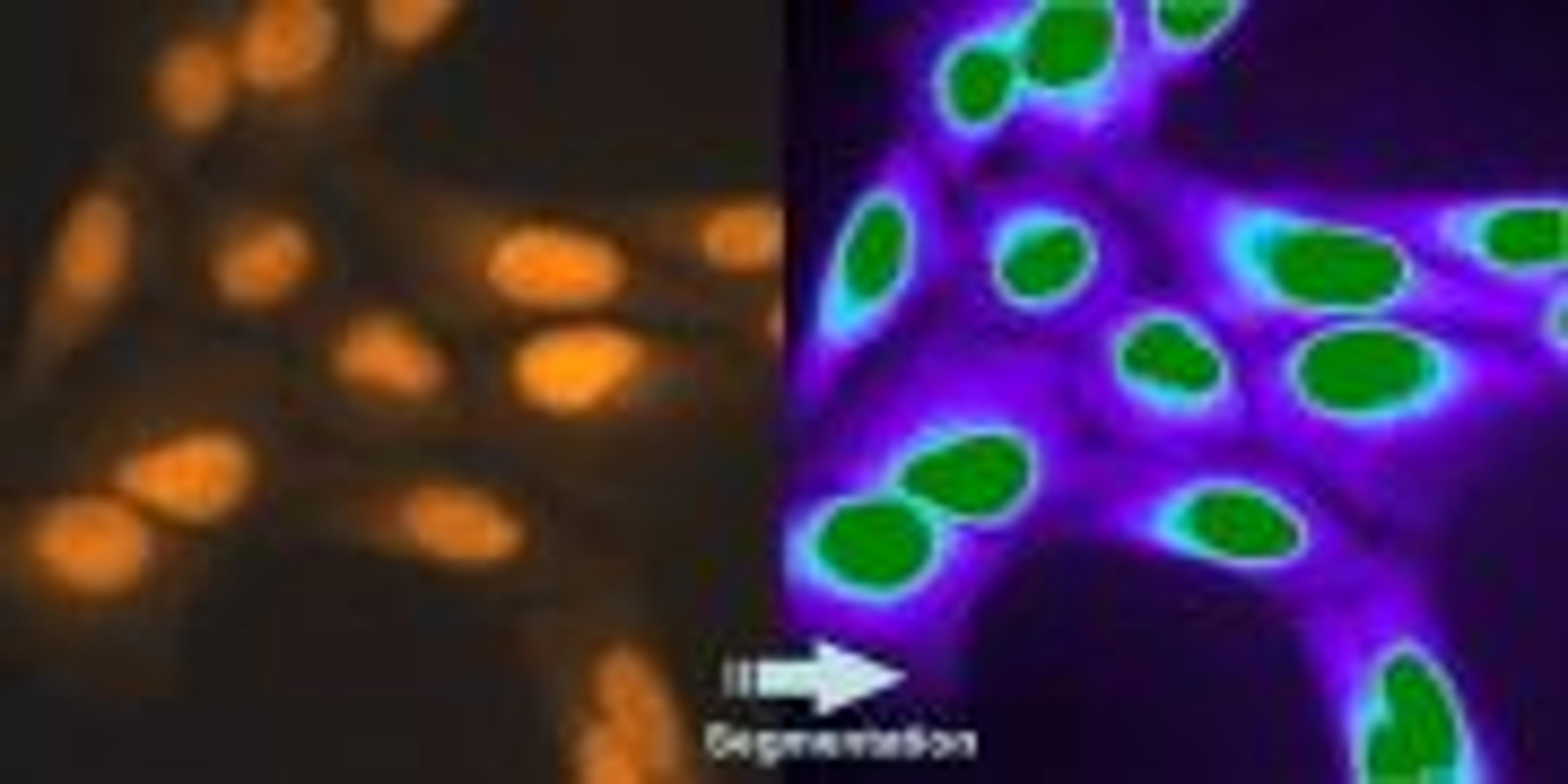
The far-red viability dye DRAQ7 has been used to monitor cell viability in pancreas tissue slices. This novel application has been developed by Marciniak, et al. (PloS One 8.11 (2013): e78706) allowing them to study endocrine and exocrine cell physiology in situ. DRAQ7 enables, for the first time, such applications due to its verified ultra-low toxicity as validated by Akagi, et al. (Cytometry Part A. (2013) 83.2: 227-234).
No-Lysis Flow Cytometric Analysis of Dyserythropoiesis
A recent Leukemia paper by Mathis, et al. (Leukemia 27.10 (2013): 1981-1987) demonstrates use of CyTRAK Orange to allow lysis-free analysis of whole bone marrows. This “live nucleated cell” gate was combined with BV421, FITC, PC-7, APC and APC-AF700 coupled antibodies and analyzed on a Beckman Coulter Navios. Importantly, forward and side scatter parameters are preserved using CyTRAK Orange. CyTRAK Orange is not excited by violet or red laser lines and does not overlap with the FITC channel allowing for multi-color panels whilst avoiding time-consuming lysis procedures which may causes uncontrolled cell losses.
Improved MK Quantitation in Bone Marrow
The far-red, live-cell permeant DNA dye DRAQ5 has been combined with thrombocyte and lineage markers for analysis by Amnis Imagestream imaging flow cytometry to study megakaryocytes (MK) in bone marrow. This approach improved quantitation of MK, further extended by polyploidy distributions and a direct indication of MK cell exhaustion, which appeared to be linked to the highest polyploidies. Using DRAQ5 this was all possible without any cell fixation, avoiding unnecessary sample preparation steps. See Niswander, et al. (Cytometry Part A (2014) 85.4: 302-312).