Free Webinar: Achieve Your Automation Potential for Released N-Glycans
Powerful technologies to streamline your N-glycan profiling
1 Nov 2017
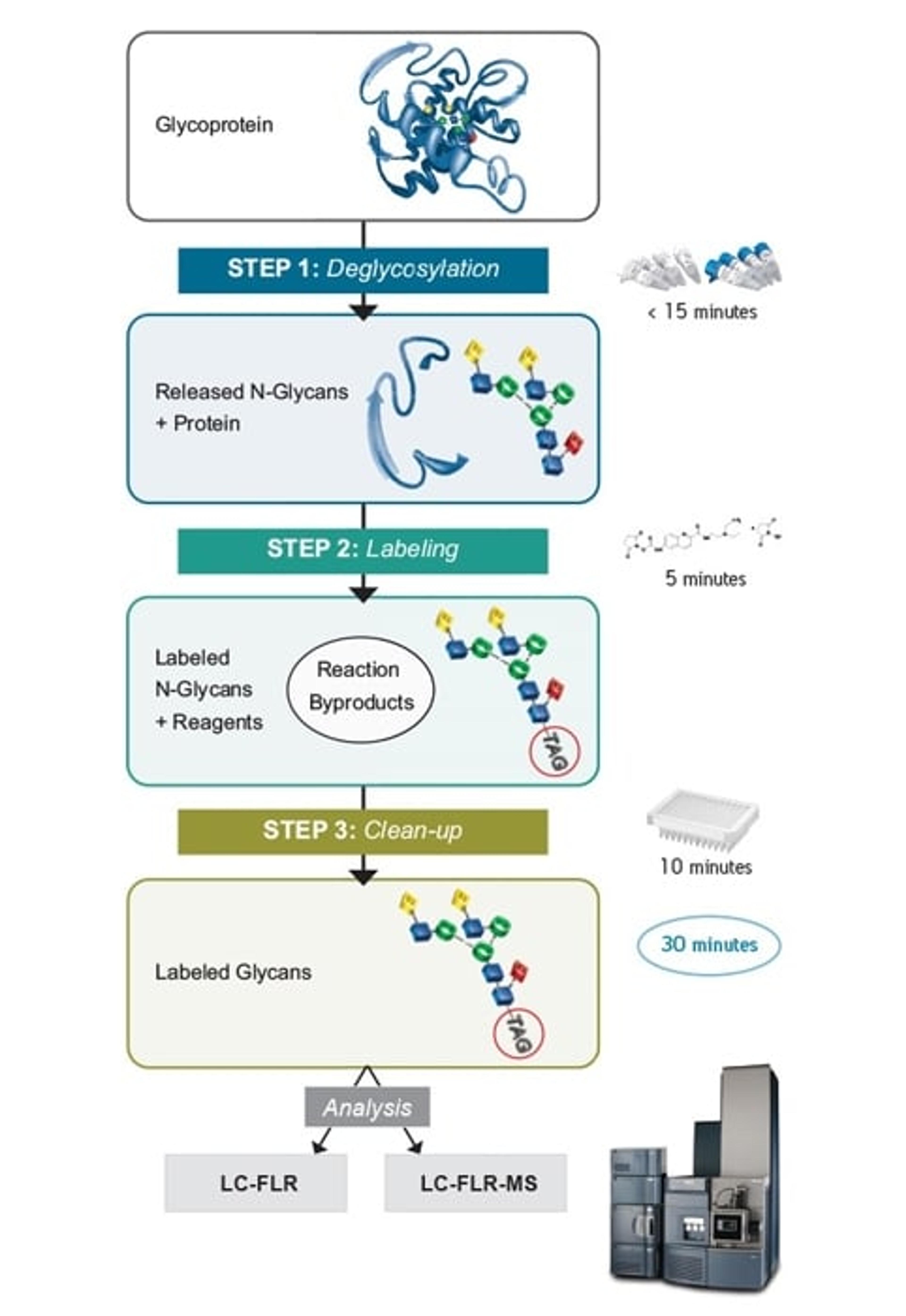
The N-glycan profile of a biopharmaceutical is commonly defined as a critical quality attribute, as it can be a measure of product efficacy and safety, and can also be used to monitor the consistency of manufacturing. The Waters GlycoWorks RapiFluor-MS N-Glycan kit provides a rapid, efficient, and reproducible protocol for this historically complicated method. As sample numbers increase, however, the time of manual preparation can add up. Automation, therefore, would have huge potential, could save analyst time and further increase laboratory efficiency.
In this webinar, experts from Waters Corporation, Phillip Lambert, senior development chemist, and Danielle Cullen, product development chemist, will explore the benefits that automation can bring to this workflow, including the need for specifically designed consumables that enable information to be translated seamlessly into the automation platform.
Register today to learn about:
- Automation options of the GlycoWorks RapiFluor-MS Kit for <8 to 96 samples
- Robustness testing that goes into verifying consumables for automation
- A platform agnostic approach and how you can use your platform with Waters consumables
- A 10-minute analytical method that allows rapid monitoring of released N-glycan profiles
You will also have the chance to ask questions for the Q&A session.
The webinar will take place on November 13, 2017 at:
- 16:00 GMT
- 11:00AM EST
- 8:00 PST
- 17:00 CET
If you can't attend the live event, please register to gain access to the webinar in your own time.