From Whole Well to Single Cell – Enhancing the Development of Cancer Biosimilars with Advanced Flow Cytometry
Scientists reveal how plate-based flow cytometry is advancing cancer biosimilar development through single-cell analysis
29 Apr 2019

Biosimilars are large, complex biological therapies with a very closely related structure, function, purity and potency to an already existing ‘reference’ drug, developed for when the current patent comes to an end. Throughout 2018, the market expanded significantly, and almost 50% of those currently approved are for cancer - including Trastuzumab’s biosimilar, Ogivri, for stomach and breast tumors.
Now, pharmaceutical companies across the globe are positioned to progress hundreds more through the drug development pipeline, thus helping to address the rising costs of cancer therapies. Yet, with increasing pressures to accelerate drug discovery, the biosimilar market is in need of more robust comparability assays, that are compatible with high-throughput formats.
Based in Glasgow, UK, Sartorius Stedim Biotech have focused on providing novel solutions to the biosimilar market for several years. To find out more, SelectScience speaks with assay development scientists Katie Chapple and Ben Tyrrell, both experts in the field.
Here, they discuss how the latest advances in flow cytometry technology are enabling biosimilarity binding studies to be conducted at the single-cell level - providing unprecedented experimental resolution at the same rate as conventional plate-reader assays.
SS: Why are biosimilars so important in cancer drug discovery, and what’s the reason for this sudden wave of interest?
BT: There are lots of opportunities for biosimilars to enter the market right now, many first-generation monoclonal antibodies are reaching the end of their patents – opening new avenues for drug discovery. Ultimately, the development of biosimilars will help to improve patients’ lives by addressing the rising cost in cancer therapies, making them more widely accessible.
SS: What challenges still exist in developing biosimilars?
KC: One of the primary challenges, is the characterization of the biosimilar in a timely, and physiologically-relevant, manner. Small structural changes can in fact lead to large functional changes, so it’s important to carefully evaluate biosimilar activity in comparison to the marketed reference drug in a wide range of orthogonal methods.
Traditional 96 or 384-well plate-based assays have revolutionized the drug discovery process, but even these can be limited to single well averages of clonal populations. Whilst the single-cell detail provided by flow cytometry can deliver an even greater level of resolution, until now, the technology has been limited by low sample throughputs, and difficulties in translating workflows from adherent to suspension cell formats.
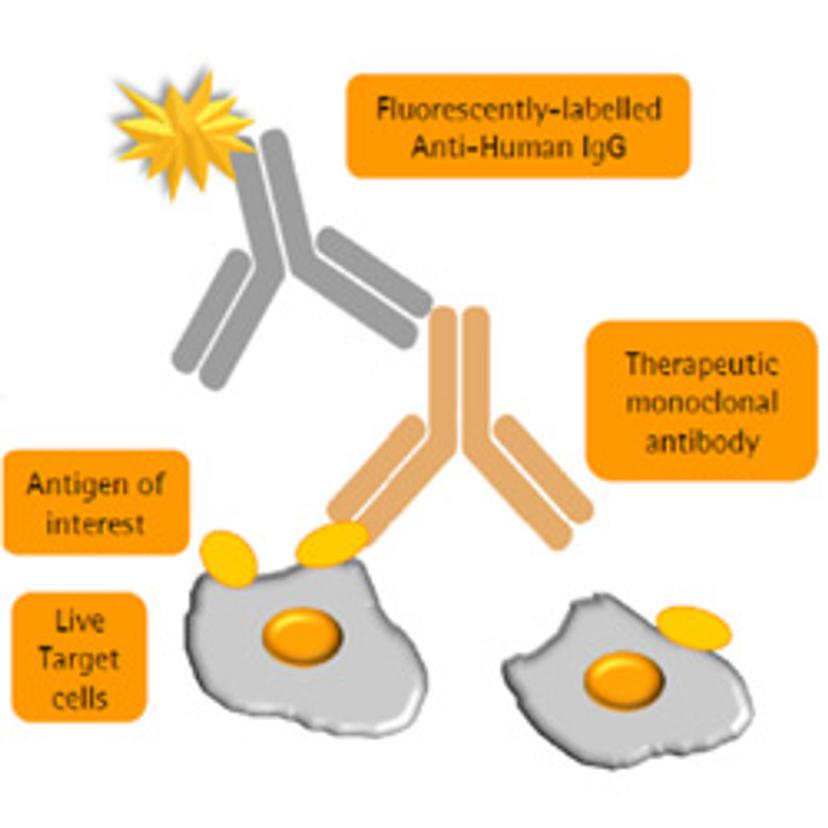
SS: How is your work helping to address these challenges?
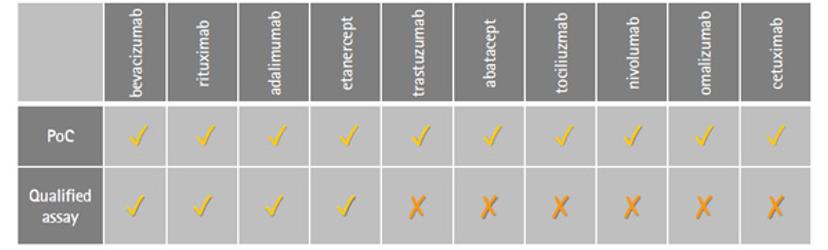
BT: Within our research park here in Glasgow, we have three focus areas – cell bank manufacturing, biosafety and bioanalytical testing. Katie and I work in technical development for the bioanalytical stream, within which there’s a wide knowledge base to produce off-the-shelf assays for testing monoclonal antibodies, and other biologics. Within our biosimilar services, our efforts in flow cytometry have been driven towards the development and qualification of flow-cytometry based binding assays for comparative studies of existing drugs — including Rituximab, Humira, Enbrel and Avastin — with potential biosimilars. The VEGF binding assay for Avastin biosimilars was one of the first assays we completed in the flow cytometry format; it was such an interesting study to be part of and has paved the way for more accurate similarity studies to be performed.
SS: Talk me through the Avastin/VEGF study – the rationale, experimental setup and key findings
KC: This was an assay we were both involved in developing and qualifying. The overarching goal was to demonstrate the transition of one assay, intended to characterize VEGF binding, from the traditional plate reader format through to flow cytometry. For this purpose, we brought in the Intellicyt® iQue screener PLUS.
VEGF is a protein that is normally secreted but remains associated with the cell surface. When bound to one of its receptors, it can mediate signaling that initiates blood vessel formation – a process known as angiogenesis. In certain cancers, VEGF is overexpressed to potentiate the angiogenic activity that is required for tumor survival. Avastin is a monoclonal antibody drug that binds to VEGF to halt hyperactive angiogenic pathways and, with its patent expiry set for 2020, there is a window of opportunity for Avastin biosimilars to enter the market.
The other main motivation to develop this assay is that by using flow cytometry, we’re using live cells – a much more biologically relevant way of modeling surface receptor activity.
BT: It certainly wasn’t straight forward, it’s a very different way of thinking - approaching an assay with live cells and suspension formats. This adds variability to your approach. That said, once we’d completed the work, the result was fantastic. We delivered a robust assay with accurate dose-response curves that was compatible with challenging cell lines, and a high-throughput platform method. Plus, we went on qualify it. We now have four proof-of-concept assays that are commercially available.
SS: How did the Intellicyt® iQue Screener PLUS facilitate this work?
BT: The iQue is a remarkably intuitive piece of equipment, I’d never done flow before my position here, but I was able to pick it up and get on with it. Fundamental to our work, was its ability to analyze plates, and analyze plates quickly. The number of samples we needed to run was huge so that was probably the greatest benefit.
One critical aspect to consider during pre-clinical research is the quantity of sample required for your assays. With the iQue, we can run assays in microliters rather than hundreds of microliters – which gave us so much more flexibility to optimize the process. This also meant, crucially, we could work with a batch of cells rather than successive passages. It’s also got a plate shaker and probe wash station, and each of these elements can be tweaked to engineer the perfect assay.
KC: Flow cytometry has been the main thread of my research, so I’ve experienced a number of cytometers over the years. In addition to Ben’s points, another main benefit I see with the Intellicyt® iQue Screener PLUS is the ability to auto-compensate your data, especially when building large multiplex panels for analysis.
SS: What does the future look like for the field of biosimilars, and what projects will you be embarking on over the next year?
KC: My work is going to involve even more complex assays on the Intellicyt® iQue – the main focus of my research over the coming months will look at qualifying T cell activation assays and mixed lymphocyte reaction assays – multiplexing with all 13 channels of the iQue Screener PLUS.
BT: I’m also currently working on multiplex assays to look at ADCC, antibody dependent cellular cytotoxicity, and ADCP, antibody dependent cellular phagocytosis, in the same assay. Unless you use flow cytometry, the only way to read out ADCP is by utilizing a reporter cell line which makes the assay non-physiological.
On the biosimilar side of things, we’re looking forward to continuing working with a lot of interesting clients and molecules, and seeing our work directly impact cancer therapeutic regimens. It’s a fascinating field to be part of, as many of the big blockbuster drugs are reaching the end of their patents.
We’ve also launched our NBE (new biological entity) platform service this year – if a client has a molecule that they think will bind a target, our site-wide expertise and GxP level facilities can be utilized to develop custom solutions using our wide range of platform methodologies.
