High-pressure systems and synthetic biology to tackle global warming
In a guest editorial from H.E.L Group, explore how pressurized bioreactors and synthetic biology can mitigate the dangerous effects of climate change
12 Jun 2024
Mario Toubes-Rodrigo, Application Leader, H.E.L Group
Integrating advanced synthetic biology techniques with high-pressure fermentation technology offers an innovative approach to carbon utilization, which can help us address some of the most urgent issues lurking on the horizon ahead.
Under pressure
We are living in unprecedented times. For the first time in recorded history, in the last year, our ocean temperatures have set new records every single day.1 The reason behind this has been the combination of certain climatic phenomena, such as El Niño, with the accumulation of greenhouse gases (GHG) in our atmosphere.
The accumulation of such chemicals results from distrupting the natural carbon biogeochemical cycle. In this, inorganic carbon, such as CO2, can be fixed into organic matter due to the activity of certain organisms such as plants, algae, cyanobacteria, and, to a lesser extent, other microorganisms. This organic matter can be stored as “recalcitrant” – very difficult to degrade – carbon, such as lignin, one of the main components of wood. Organic matter can also be buried and transformed into other substances, such as coal or oil. Naturally, this buried carbon would remain stored for hundreds of years until it becomes part of the mantle and escapes to the atmosphere as part of volcanic eruptions.
However, since the dawn of humanity, this cycle has been broken, and since the Industrial Revolution, the release of C into the atmosphere has increased exponentially. As a result, between 1880 and 1980, the temperature on Earth increased by 0.07°C per decade, increasing to a whopping 0.18°C per decade since 1981. We have experienced this anomaly firsthand: the unprecedented heatwaves in the Pacific Northwest in 2021, London hitting 40°C for the first time in 2022, and July 2023 being declared the hottest month ever recorded on Earth.
Synthetic biology in a warming world

Autotrophic organisms can capture inorganic carbon (especially CO2) and incorporate it into organic matter. Photosynthesis is a well-known example, but many organisms can also fix carbon using light-independent processes (chemolithotrophy). Instead of using solar energy to power their metabolisms, they can harness chemical reduction-oxidation reactions to power this. Evolution has equipped chemolithotrophic organisms with various routes to achieve this.2
Utilizing autotrophic organisms is becoming an emerging trend. This seems to be a perfect solution. We have an excess of raw material, in this case, CO2, which is also a cheap resource. The carbon fixed by the cell can take the form of high-value products. For example, organisms such as different species of Clostridium have been used to generate biofuels, including ethanol or 2,3-butanediol from synthetic gas (syngas), a bioproduct from industrial processes. Other biofuels, like butanol, hexanol, and hexanoic acid, can be produced by other species, such as C. carboxidivorans. However, an even more intriguing option is the capacity to re-route the carbon flux in the cells using genetic engineering. For instance, E. coli, the most used model organism in microbiology, is a very well-known heterotrophic organism. However, its modification, adding some of the genes encoding for the enzymes involved in autotrophy (e.g., phosphoribulokinase and RuBisCO), has resulted in variants able to produce high concentrations of biofuels taking CO2. Additionally, photosynthetic organisms can be modified, resulting in a higher rate of CO2 capture.
Success in spite of the pressure
Despite the problematic abundance of CO2, its solubility in water can be limiting for its effective use in industrial processes. Microbial cells can only access this gas when dissolved in water. However, gases in general, and CO2 in particular, are sparsely soluble. Different parameters influence the capacity of this gas to dissolve in water. Nevertheless, striking the right balance between the conditions that increase the concentration of gases in the liquid medium and the ideal conditions for cell growth is challenging. Whereas lower temperatures favor solubility, they also decrease the metabolic activity of most microorganisms used in industry. pH has a similar impact. The highest solubility occurs at lower values, and the equilibrium is displaced towards ionic forms (bicarbonate and carbonate), reducing carbon bioavailability. However, acidic conditions can be detrimental for mesophilic microorganisms.
Pressure can be an unexpected yet intriguing ally in the search to increase the effective amount of soluble CO2 available for microorganisms. Whereas high pressure can be deleterious for microbial proliferation, most microbial cells can withstand moderate pressure conditions. Conversely, it is well established that pressure increases the solubility of gases (including CO2). Although still in its infancy, preliminary research has shown how beneficial pressurized reactors can be for gas fermentation.3 However, it was highlighted that further research is required since pressurized reactors are hardly available.
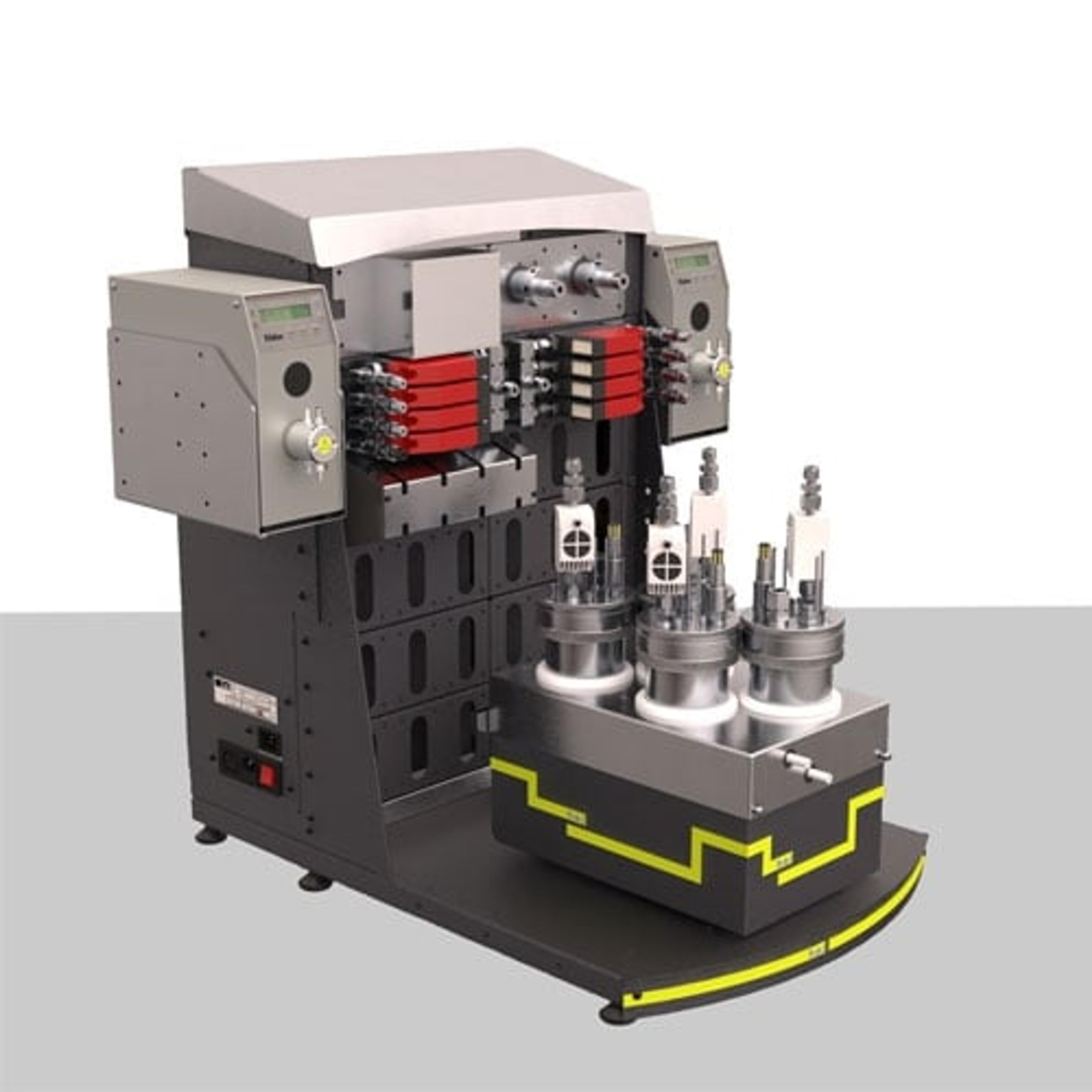
Nevertheless, there is at least one pressurized bioreactor system, H.E.L Group’s BioXplorer 400P. This model has been used in several applications involving headspaces containing H2 and CO2. Garrigues et al., 2019 were able to increase the production of isopropanol one order of magnitude to previous studies while maintaining safe O2 concentration (<2%).4 Tarraran et al., 2023 produced acetone and acetic acid from modified Acetobacterium woodie using CO2 and H2,5 whereas Regis et al., 2024 used Thermoanaerobacter kivui.6
What the future holds
As humanity faces an increasingly challenging situation, high-pressure systems, and synthetic biology become promising solutions to mitigate the dangerous effects of climate change. The innovative use of pressurized bioreactors increases the solubility of CO2 and maximizes the efficiency of carbon capture.
The preliminary successes observed with pressurized systems, such as those enabling the production of biofuels and other bioproducts, are a beacon of hope. These systems demonstrate that microorganisms can thrive under pressure, turning potent GHGs into valuable resources. This not only helps in reducing atmospheric CO2 levels but also contributes to a sustainable bioeconomy. Integrating advanced synthetic biology techniques with high-pressure fermentation technology offers an innovative approach to carbon utilization, which can help us address some of the most urgent issues lurking on the horizon ahead.
This guest article was written by Mario Toubes-Rodrigo, Application Leader, H.E.L Group
References
1. McGrath, M., Poynting, M. & Rowlatt, J. (2024) Climate change: World’s oceans suffer from record-breaking year of heat, BBC News. Available at: https://www.bbc.co.uk/news/science-environment-68921215
2. Kajla, S., Kumari, R. & Nagi, G.K. Microbial CO2 fixation and biotechnology in reducing industrial CO2 emissions. Arch Microbiol 204, 149 (2022). https://doi.org/10.1007/s00203-021-02677-w
3. Van Hecke, W., Bockrath, R. & De Wever, H. Effects of moderately elevated pressure on gas fermentation processes. Bioresource Technology, 293, 122129 (2019). https://doi.org/10.1016/j.biortech.2019.122129
4. Garrigues, L. et al. Isopropanol production from carbon dioxide in Cupriavidus Necator in a pressurized bioreactor. New Biotechnology, 56, 16–20. (2020). https://doi.org/10.1016/j.nbt.2019.11.005
5. Tarraran, L. et al. High-pressure fermentation of CO2 and H2 by a modified Acetobacterium woodii. Journal of CO2 Utilization, 76, 102583. (2023). https://doi.org/10.1016/j.jcou.2023.102583
6. Regis, F. et al. Screening of conditions for the acetic acid production from H2 and CO2 by Thermoanaerobacter Kivui in a pressurized stirred tank bioreactor. Chemical Engineering Journal, 485, 149685. (2024). https://doi.org/10.1016/j.cej.2024.149685