High-temperature ATR measurements of soaps and foodstuffs
Discover how the Quest™ Heated Puck from Specac has been used to analyze physical separation and phase transition temperatures of soaps and foodstuffs
17 Jan 2022

Infrared spectroscopy reveals details about molecular bonding and structure by measuring the energy absorbed by the vibrational modes of molecules. Chemists are able to identify functional groups in the molecule via the characteristic peaks produced by them in an infrared spectrum. This is usually straightforward for pure samples or simple mixtures of known substances. For more complex matrices, such as those found in food and other bio-based materials, the task is more difficult and requires additional insights from other techniques (such as mass spectrometer or NMR spectroscopy).
By varying the temperature of the sample, one can observe peaks growing and decaying in intensity, broadening, or shifting in position to higher or lower frequencies. All these are indicators of structural change in the sample and can be correlated with interactions between the sample components, phase changes within the material, or conformational changes in individual molecules such as proteins.
Specac has demonstrated the use of the Quest Heated Puck in two different temperature-controlled experiments: one looking at the physical separation of butter into solid and liquid phases, the other for analyzing the phase transition temperature of a soap/stearic acid system.
Colloidal mixtures
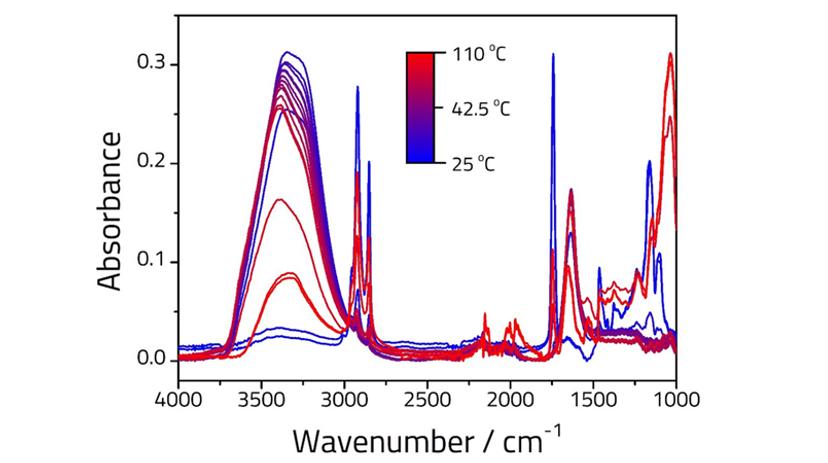
Milk is a colloidal mixture of water and fat particles surrounded by a shell of proteins, which stops them from congealing into larger particles. These proteins include lactose and casein and can be quantified by FTIR1. Butter is produced by churning milk to break down the protein shell, allowing the fat to coagulate into large butter particles suspended in a buttermilk liquid phase containing the lactose proteins. On heating butter to its melting point, the emulsion separates into a fat phase on top and a water phase containing the milk solids on the bottom. This process is known as the clarification of butter.
The clarification of butter into two phases can be seen clearly when heated from room temperature to 110 °C using a Quest™ heated ATR puck. Figure 1 shows the corresponding FTIR spectra. At low temperature (darkest blue line) the peaks associated with the oil component of the emulsion are clearly visible (saturated CH stretches, 3000-2800 cm-1, C=O stretch at 1740 cm-1 and fingerprint region, 1500-1000 cm-1), as well as small peaks associated with the water component (3390 and 1630 cm-1). As the sample is heated the emulsion separates into two phases, with the oil phase (which floats to the top) disappearing from the spectrum due to limited depth of penetration2.
Acid-soaps
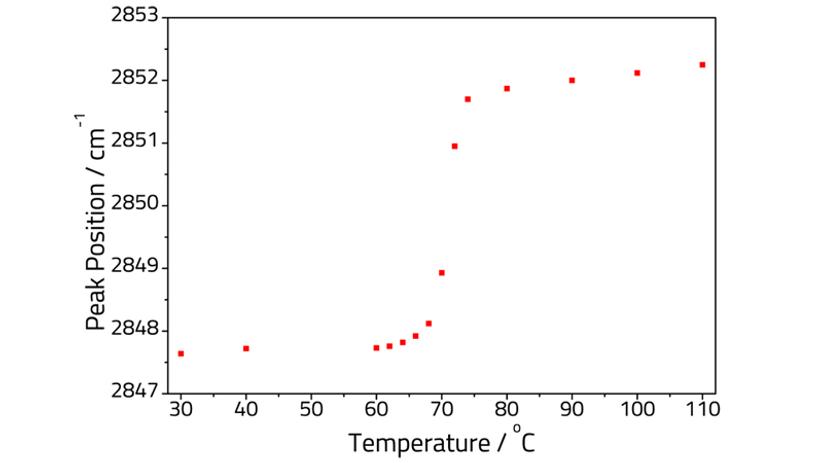
Acid-soaps are a class of commercially produced compounds used in soap bars, cleaners, and cosmetics. Pudney et al. reported an ATR study of the phase behavior of stearic acid-triethanolamine (TEA) soap, from 0 to 100% neutralization of the acid3. Specac was able to replicate part of this study using a heated Quest™ ATR. In their work Pudney et al. were able to construct a phase behavior diagram from the 20 sample concentrations they had analyzed; at 85% acid neutralization the authors reported that the CH2 stretching vibrations decreased in intensity, shifted in position, and broadened upon heating.
Figure 2 plots the variation of the peak position of the CH2 stretching frequency with temperature. The overall trend of the graph shows a good match to the previous work3. Both plots show the peak shifting slowly from ca. 64 to 70 °C, corresponding to a multiphase system. Above 70 °C the peak frequency rapidly increases in both experiments corresponding to the transition to a liquid crystal phase.
Summary of key features of the Quest™ Heated Puck
- Heat liquid and solid samples up to 110 °C
- Accurate and stable temperature measurement via 4-wire RTD sensor
- Superior physical and chemical robustness provided by monolithic diamond ATR crystals
- Setting and logging temperature made simple by PC software and compact USB interface
- Retrofits to all Quest family accessories
References
Specac Application note: AN16-02 Analyzing Milk with the Pear
Specac Technical note: TN21-02 ATR Penetration Depth
Pudney P.D.A., Zhu S., Mutch K., Phys. Chem. Chem. Phys., 11, (2009), 5010–5018. DOI: 10.1039/b819582j

