How 3D-Based Image Analysis is Revealing Complex Cell-Cell Interactions to Advance Immuno-Oncology Research
Scientists from OcellO Netherlands discuss their approach to 3D analysis of interactions between tumor and immune cells
26 Jun 2018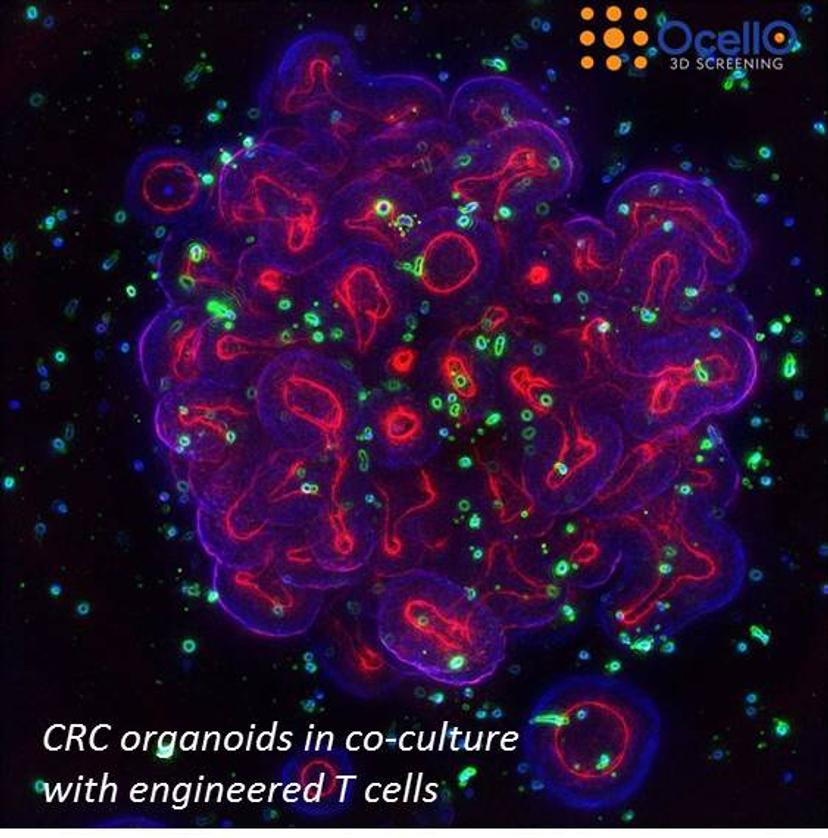
3D screening provides a more physiologically relevant platform that provides rich data, essential for faster drug discovery. In this interview, we hear from Dr. Lidia Daszkiewicz, head of immuno-oncology at CRO OcellO B.V, and Dr. Kuan Yan, its chief informatics officer, on the advantages of 3D image analysis of 3D assays and how this will help to advance the development of immune-oncology drugs.
SS: Please introduce yourself and tell us a bit about your role at OcellO
Kuan Yan: I'm the chief informatics officer (CIO) of OcellO B.V. Our company is a Netherlands-based contract research organization (CRO) specialized in drug screening using cell lines, organoids and primary human material. My role in the company is to provide analysis solutions for drug screening procedures, image analysis algorithms, machine learning and artificial intelligence.
Lidia Daszkiewicz: I'm head of immuno-oncology at OcellO. I run the department responsible for testing immunotherapy for different customers in the immuno-oncology field. I design the immuno-oncology assays, discuss projects with customers and oversee projects.
SS: Tell us why you chose to focus on 3D cell cultures and 3D imaging
KY: We’ve focused on 3D cell cultures and 3D imaging as tissues cultured in a 3D environment more closely resemble tissues in humans phenotypically, functionally and with respect to gene expression profile and drug responses compared to conventional 2D monolayer cultures. Compared to mouse models, it is cost efficient, the experiment cycle is much shorter (weeks instead of months) and we can perform an almost unlimited number of tests, requiring only a tiny amount of compound. If you want to test drugs using mice you have to sacrifice hundreds of mice to get a reasonable readout, but for us it's just a single plate, so we can do this in 30-40 minutes of analysis and then we get a readout.
LD: As Kuan says, our assays and models are more clinically and physically relevant and this is thanks to the improved morphology of tumors grown in 3D and an improved gene expression profile. Of course, there is a difference between the cells growing in 2D on flat plastic or floating as a spheroid and embedded in a natural extracellular matrix, where cells receive all kinds of cues that drive the formation of these 3D structures, which is critical for their functionality. We've seen that, apart from the fact that these cells look much more physiologically relevant, they are really close to the histology that you see in patient biopsies, and they are also more functional. So that's the most important reason.
Another reason is the cost and time efficiency – so there has to be the right balance between the complexity of the screening platform and its relevance. We see a lot of technologies such as genomics and proteomics, but in general these are basically measuring expression of certain surrogate reporters, not actually measuring how tissues respond to compounds in an environment that is similar to the human body. So, by using 3D cell culture, we're more or less putting the tissues in an environment where drug response data will be more predictive of patient response compared to DNA profiling or target-based analysis.
SS: Why is 3D based image analysis important?
If we talk about immuno-oncology, then of course the future is bright.
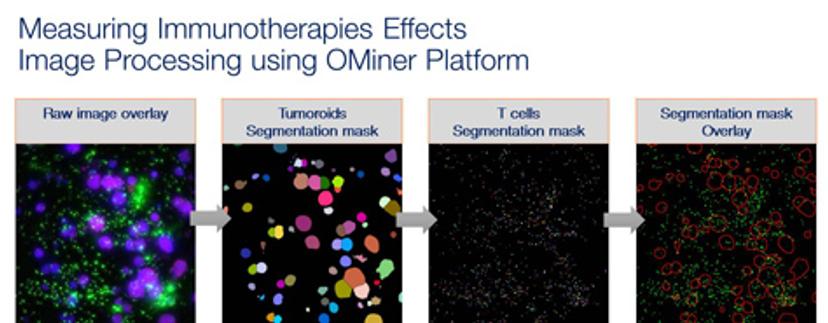
KY: For 3D analysis, sometimes a particular phenotype resulting from treatment can only be observed correctly from 3D tissue cultures. For example, we measure T-cell infiltration in tumoroids. If you measure such information from the 2D culture, firstly, you won't be able to observe infiltration because the tumor tissues in the 2D assays become a monolayer. Secondly, most of the time when people do these kinds of infiltration assays, they do projections of the image 3D image stack, which introduces a false reading in the amount of T cells inside the tumoroid. These kinds of readouts can only be observed from a true 3D-based analysis. So basically, the reason why we always use 3D image analysis, is that you can see and measure a lot more compared to more simple visualizations and biochemical assays – it’s essentially multiplexing.
SS: Which technologies are causing the most innovation in your work?
LD: Lab automation is of crucial importance for our particular platform and also in general, because in pharma and biotech, high throughput is important. So, whatever helps to scale up the experiment and get the most data out with less input is of course the greatest advancement. Automation doesn’t just improve throughput, it also improves reproducibility. Our platform is fully automated — we use liquid handlers and they are quite a help. Our 3D platform is very advanced and we continuing to develop it.
KY: On the other hand, once we collected images, it requires the use of cloud-based computation, large data analysis, machine learning and database storage technologies. There are commercial solutions available, but what we realized is that they're often designed to serve a single purpose, and it is quite a limited purpose. So, what we found out is that you need a very strong integration between the biological side and computer science to produce the most optimal solution. That's the reason why we produce our own image analysis and automation platforms.

SS: Tell me about the talk that you're presenting at SLAS Europe
KY: Our talk will focus on the image-based immuno-oncology assay using 3D gel-based cell cultures. The talk is mostly focused on presenting how we prepare the experiment, how we do the imaging and image analysis – what kind of readout can we extract from the image analysis and what kind of functional end-point readout can we get from the analysis, and of course, a couple of examples to show how we can we distinguish T cell-tumor interactions in different scenarios.
LD: What we hope to get out of this presentation is to make the audience aware of how the 3D environment is important for studying immuno-oncology drugs. It’s not just the improved biology, but if you need to get spatial information, like the relation of immune cells to tumoroids, infiltration and all kind of interactions, you need to have the experiment done in 3D and you need to have the analysis done in 3D. As Kuan said, image compression – basically getting information out of 2D after performing experiments in 3D – then we are missing out on a lot of the details. So, we are trying to convince users of how important it is to preserve 3D environment for both biology and readouts.
SS: What do you see for the future of your work and its implications on healthcare?
LD: If we talk about immuno-oncology, then of course the future is bright. Immuno-oncology is already a high-volume market and growing, and there are predictions that in approximately six years, immuno-oncology will be able to cure around 60 percent of cancer, so that gives you the scale of importance of the immuno-oncology field. As this is growing, the tools also need to be continuously developed to help immuno-oncology drug developers bring those treatments to market. So immune-oncology will be important, and our company will continue to grow this branch of our service offering.
KY: In terms of software, we continue to develop our analysis capabilities. Right now, we have a collection of analysis pipelines for 3D PDX models, organoids, and all kinds of primary human materials: branching structures for invasive models, resistant models, human iPS neurons, and even bone tissues. So, we're continuing to improve the capabilities and scalability of our platform to meet the increasing demands on quality and throughput of our customers and academic partners.
Catch Kuan Yan’s presentation at SLAS Europe 2018 in the Next Generation Analytics session at 8.45 am on Friday, June 29.
Plus, visit SelectScience at booth #414, June 27-29, to meet the team and pick up a free gift.
