How AI is advancing neurodegenerative disease research
In this guest editorial, explore how artificial intelligence is becoming a powerful tool for drug discovery
4 May 2021
As part of the SelectScience Advances in Drug Discovery Special Feature, guest editors Dr. Matthias Fassler, Genedata, and Daniel O’Connor, Molecular Devices, take a look at how the combination of automated imaging and deep learning is addressing research challenges and highlight how this approach helps accelerate drug development.
The prevalence of neurological disorders is on the rise, with Alzheimer’s and Parkinson’s cases estimated in the millions. To combat this trend, many researchers are turning to artificial intelligence (AI)1, which has proved useful for identifying complex disorders and aiding in optimized interventions. AI technology predicts cognitive impairment and anticipates how severely motor skills might decline over time thereby helping to accelerate patient diagnoses and improve prognoses for neurodegenerative diseases2. Scientists can also employ AI to discover potential drug and biological targets that could lead to better treatments.
In a recent study, researchers at Mount Sinai Hospital developed an AI platform to detect a range of neurodegenerative diseases in human brain tissue samples. Their work is expected to help scientists develop targeted biomarkers and therapeutics3, leading to more efficient and accurate diagnoses of complex brain diseases. These exciting advancements are unleashing an ability to design therapies tailored for individual patients, ultimately improving patient outcomes.
AI and deep learning set new benchmarks for imaging analysis
To understand and identify trends and markers in neurobiology research, scientists must collect, analyze, and characterize massive amounts of data. This requires preparing complex assays, performing high-content multi-parametric analysis, and interpreting large, complicated datasets to determine the neurotoxicity levels of various patient treatments.
In the biotech industry, organizations harnessing and analyzing big data faster and more effectively can deliver the right diagnoses and therapeutics to patients quickly. New collaborations among innovative technology providers are enabling more biopharmaceutical laboratories and research leaders to extract data-rich insights while offering end-to-end automation with optimized image analysis capabilities.
Molecular Devices and Genedata have come together to demonstrate machine learning methods for analyzing morphological endpoints in complex cell models by integrating deep learning-based software (Genedata Imagence® 2.0) with an automated, high-content imager (ImageXpress® Micro Confocal High-Content Imaging System). The combination, which delivers incremental machine learning and knowledge preservation, analyzes complex phenotypic assay formats without extensive statistical models or expert input. Now, biopharmaceutical companies can quickly and effortlessly speed development and broaden the rollout of imaging assays in their R&D workflows.
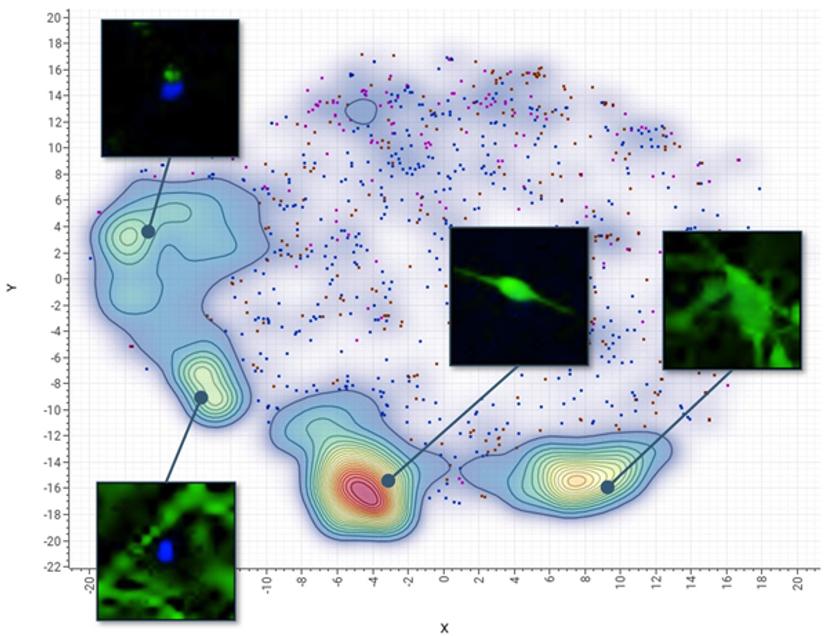
Advantages of an AI-based approach
To illustrate the advantages of combining high-content imaging with an AI-based analysis platform when quantifying complex biological phenotypes, Molecular Devices and Genedata developed a cell-based test for neurotoxicity evaluation using induced pluripotent stem cell (iPSC)-derived neuronal cells.
The work tested a set of neurotoxic compounds with suspected toxic effects on the nervous system, including established drugs to treat cancer, as well as environmental substances. After iPSC-derived neurons were treated with compounds for 72 hours, they were imaged with a high-content imager and the data was then analyzed using convolutional neural networks (CNNs).
A CNN is essentially a machine learning model that takes raw images as input, assigns importance to various aspects within the images, and learns to differentiate one from another. CNNs are more efficient than the conventional phenotypic analysis approach where readouts can include characterization of complex neurite structures – such as neurite outgrowth, branching, number of processes, and cell viability – making it time consuming and challenging to analyze data at scale. Instead, they defined a set of representative images for reference phenotypes such as those induced by control treatments. This so-called training set enabled the CNN to automatically recognize criteria separating the phenotypes, eliminating the need for analytic software experts who are required in conventional image analysis.
The test defined four reference phenotypes in the CNN that reflected different states of stem cell differentiation and cell damage. With AI, 1,000 cells per phenotype were sampled in just a few minutes versus the hours typically required with conventional phenotypic analysis. Instead of presenting multiple readouts that had to be further analyzed in a multi-parametric fashion, the deep learning-based approach provided a more integrated assessment of neurotoxicity.
The trained CNN predicted neurotoxicity with the same sensitivity as a conventional solution, finding some substances caused severe disintegration of neural networks and cell death while selected drugs caused mostly moderate perturbations. With dose-response information, we were also able to quickly and easily rank chemicals according to their toxicity or safety.
From beginning to end, AI-assisted image analysis was a game changer for the research team.
The future of automated image analysis
Deploying Genedata Imagence® 2.0 software with the ImageXpress® Micro Confocal High-Content Imaging System and unleashing the ability to automate CNN training for such neurotoxicity assays is a double bonus: laboratory leaders can free staff from onerous analysis and even reduce or eliminate resourcing demands for additional training or imaging experts.
The ability to automate CNNs training for such assays enables biologists to use these powerful tools without any expert knowledge in image analysis, thus leading to tremendous time savings during assay development. As improved high-content imaging generates more data, deep learning will be critical to R&D by enabling faster, cheaper, and more efficacious drug discovery. While research in neurodegenerative diseases has made enormous strides in recent decades, AI and deep learning will continue to provide more opportunities to understand the unknowns of degenerative diseases.
Visit the SelectScience Advances in Drug Discovery Special Feature to learn more about the technologies and methods advancing the field.
Matthias Fassler, Ph.D., product manager at Genedata, is a cell biologist passionate about bridging the gap between biologists and software developers and transferring new technologies into practical applications. Having authored and co-authored many scientific papers, Fassler leads the development of deep learning-based solutions at Genedata. (matthias.fassler@genedata.com)
Daniel O’Connor is the Vice President of Drug Discovery at Molecular Devices specializing in innovative high-content imaging technologies for screening 2D and 3D cellular disease models. With over 20 years of industry experience, he has developed an innovative application-focused approach to customer solutions. Daniel sits on the Strategic Innovation Review Board at Molecular Devices and has won numerous commercial and innovation awards throughout his career. He earned a BSc in neuroscience from University of Minnesota-Twin Cities. (dan.oconnor@moldev.com)
References:
Brasil S, Pascoal C, Francisco R, Dos Reis Ferreira V, Videira PA, Valadão AG. Artificial Intelligence (AI) in Rare Diseases: Is the Future Brighter?. Genes (Basel). 2019
Monika A. Myszczynska MA, Ojamies PN, Lacoste AMB, Neil D, Saffari A, Mead R, Hautbergue GM, Holbrook JD, Ferraiuolo L. Applications of machine learning to diagnosis and treatment of neurodegenerative diseases. Nat Rev Neurol. 2020
Signaevsky M, Prastawa M, Farrell K, Tabish N, Baldwin E, Han N, Iida MA, Koll J, Bryce C, Purohit D, Haroutunian V, McKee AC, Stein TD, White CL 3rd, Walker J, Richardson TE, Hanson R, Donovan MJ, Cordon-Cardo C, Zeineh J, Fernandez G, Crary JF. Artificial intelligence in neuropathology: deep learning-based assessment of tauopathy. Lab Invest. 2019

