How Scientists are Working to Fight the Deadliest Form of Cancer with Oncolytic Viruses
Dr. Pierre Cordelier describes how some exciting pre-clinical data suggests oncolytic viruses could tackle pancreatic cancer
27 Mar 2019

On average, patients with breast cancer have an 80% chance of survival beyond ten years. For primary pancreatic tumors, the statistic is less than 1%. Treatment options have barely inched forward over the past 40 years, due to the highly plastic nature of the disease, but a group of scientists in France are providing new hope with tumor-targeted viral therapies. In this exclusive interview with senior researcher at the Toulouse Cancer Research Center, Dr. Pierre Cordelier, SelectScience finds out how oncolytic viruses are opening up new avenues for therapy in advanced pancreatic cancer patients.
Dr Cordelier has been working in gene therapy research since early on his career – studying for a Ph.D. in Toulouse before moving to Philadelphia for his postdoctoral fellowship at Thomas Jefferson University. He has since spent 14 years at the Toulouse Cancer Research Center – part of INSERM, France’s National Institute of Health and Medical Research – where he fulfills his passion for improving the lives of patients with pancreatic cancer through viral-based gene transfer therapy.
Explaining the rationale behind their research in greater detail, Dr Cordelier says: “Almost 80% of patients with pancreatic cancer are diagnosed with metastatic disease and, at this point, surgery is not an option, unfortunately”.
“Therefore, a systemic therapy is required, such as the chemotherapy gemcitabine. The crux of the matter is, however, that tumors eventually become resistant and unresponsive. In fact, many pancreatic cancers are even innately resistant – lacking the proteins that metabolize gemcitabine inside the cell in the first place. Clinicians desperately need a more effective and more durable treatment option, therefore, and this is the pre-requisite to our research.”
Almost 80% of pancreatic cancer patients are diagnosed with metastatic disease.
During Cordelier’s Ph.D. and postdoctoral positions, he was involved in the first non-viral gene therapy clinical trial which is now in the midst of an exciting phase II clinical study.
“During this time, it was discovered that the somatostatin receptor is lacking in pancreatic tumors, and, as part of my research, we came to realize that whilst restoring expression was yet cytotoxic, it considerably limited the metastasizing capacity of the primary tumor. When we combined this with the overexpression of gemcitabine-metabolizing proteins using non-viral vector-based gene therapy, the results were dramatic. The trial is now well into phase II – patients are infused with the vector via endoscopy, in combination with chemotherapy doses, and we’re excited to see the results on completion,” says Cordelier.
He explains how, for the duration of the trial and beyond, close attention needs to be paid to the plasticity of patients’ tumors in response to this therapy, given their ability to “adapt at the speed of light”.
This unrelenting genetic adaptability of pancreatic cancer to external stressors is where oncolytic viruses come in.
“Since this clinical trial we’ve come to realize that oncolytic viruses are an even more interesting mode of gene therapy. First, the replication of viruses amplifies the therapeutic message inside the cell. What’s more, oncolytic viruses are tumor-tropic, meaning they seek out cancer cells with unique proteins expressed on their surface, and find in cancer cells the perfect “niche” to engage viral replication - due to lack of IFN response for the most part. But, until now, we haven’t been using them to their full potential – our work has brought to light their ability to kill pancreatic cancer cells in multiple ways.”
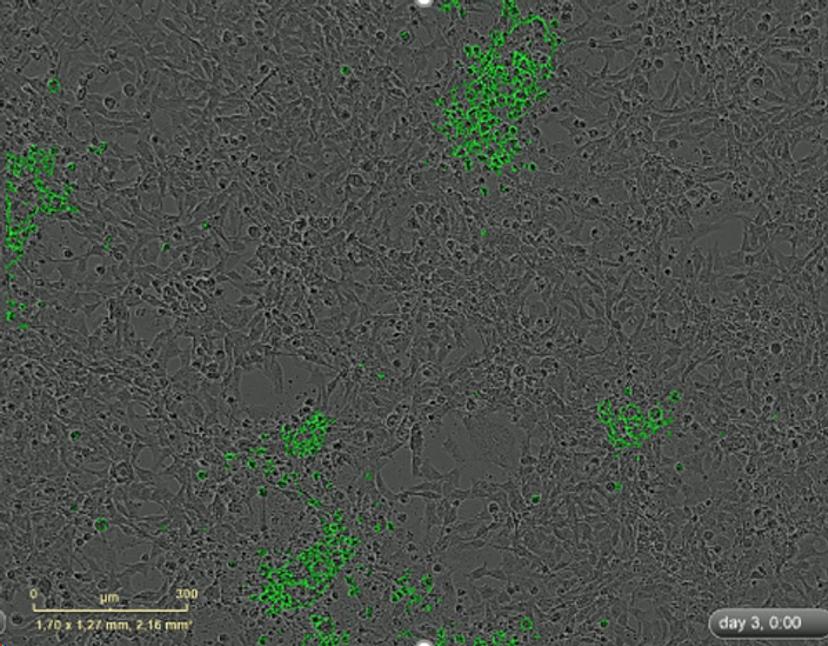
Providing a few examples, he continues: “Firstly, we can exploit their tropism further; viruses are filtered by the liver and this is the most common site of primary pancreatic metastasis. Secondly, their ability to infiltrate the tumors creates a localized site of inflammation – which we can take advantage of with additional immunotherapies. Pancreatic cancers are otherwise ‘cold’, or recalcitrant, to conventional immunotherapy.”
Working with viruses has, historically, been a challenging venture for scientists worldwide. They spread rapidly from cell to cell and testing multiple doses against varied primary cell lines can be a costly, time-consuming process. Dr Cordelier credits the speed and detail of their findings to the in-house live-cell analysis equipment they have access to.

“Viruses are hugely dynamic, so you can be too early and too late with fixed-cell fluorescent analysis, for example. With the IncuCyte we can capture GFP-expressing viruses in real time to review every stage of activity; explore multiple cell models in parallel; quickly determine which viruses work and which ones don’t; and retrieve interpretable, publication quality data. We’ve also been able to analyze the efficacy of different strains on 3D organoid models. Plus, we can send movies to our collaborating colleagues. A fresh pair of eyes can see things we can’t!”
With a body of evidence to suggest that oncolytic viruses could redress the lack of effective therapy options for pancreatic tumors, Dr Cordelier’s research group is making waves in the field. Driven by the desire to impact patient lives, he firmly believes that cooperation between scientific expertise across the world, and across varied disciplines, will drive the future of cancer therapy forward.
Do you use the IncuCyte S3 Live-Cell Analysis System in your research? Write a review to share your experiences with fellow scientists around the world.

