How spatial biology is driving tumor microenvironment research
Learn how a leading cancer researcher is utilizing the COMET™ technology in his research
11 Sept 2024

Dr. Janusz Franco-Barraza, researcher and spatial immuno-proteomics facility manager at Fox Chase Cancer Center
Understanding the complexity of the tumor microenvironment (TME) is pivotal in cancer research. This elaborate ecosystem, consisting of cancer cells, stromal cells, immune cells, and others, plays a crucial role in tumor progression and treatment response. Spatial biology, which examines spatial organization and interactions of cellular components within tissues, has emerged as a critical tool in unraveling these complexities.
Among the leading technologies in this field is the COMET™ platform from Lunaphore – a Bio-Techne brand, the only fully-automated, end-to-end solution for same-section proteomics, transcriptomics and multiomics spatial analysis.
To dig deeper into how spatial biology, and specifically COMET™, supports research in elucidating TME complexity, we spoke with Dr. Janusz Franco-Barraza, a researcher and spatial immuno-proteomics facility manager at Fox Chase Cancer Center.
The importance of spatial biology in cancer research
Dr. Franco-Barraza’s journey into the world of spatial biology began with a focus on the interactions between fibroblasts and the extracellular matrix in the context of cancer. His work has expanded to explore the dynamic interplay between stromal cells, immune cells, and cancer cells within the TME. According to Dr. Franco-Barraza, traditional research methods often adopt a reductionist approach, studying isolated cells or specific proteins. However, this method falls short in capturing the complexity of interactions within the TME.
“Now, cancer biology is moving towards a translational field where we need to understand that we are not dealing with cells in isolation but with full organs within human beings,” Dr. Franco-Barraza explains. ”Cancer cells live inside complex tissues and interact continuously with other types of cells,” This shift underscores the significance of spatial biology, which provides a holistic view of cellular interactions within their native tissue environment.
Spatial biology not only allows the mapping of relationships between cells, including their distribution within the tissue but also highlights intratumor and intertumor heterogeneity. This is critical because different tumor regions can exhibit distinct biological behaviors which in sum, impacts the responses to treatment. For instance, some areas might be more hypoxic, promoting aggressive cancer cell phenotypes, while others might be heavily infiltrated by immune cells. Understanding these spatial variations is essential for developing targeted therapies that address the specific characteristics of different tumor regions.
Hyperplex immunofluorescence with COMET™

COMET™ – a fully automated, high-throughput, hyperplex platform from Lunaphore Technologies
Among the advanced tools in spatial biology, the COMET™ platform stands out for its ability to perform sequential immunofluorescence (seqIF™) assays through staining, imaging, and elution cycles. This technique allows researchers to simultaneously detect multiple biomarkers within a single tissue section, providing a comprehensive single-cell view of cellular states and relationships. Dr. Franco-Barraza highlights several key advantages of the COMET™ platform in his research.
Flexibility and customization
One of the primary reasons for choosing COMET™ was its flexibility and open-source nature. “It’s an instrument that can be adapted to all our needs. It’s an antibody open-source technology, which is great because we have several antibodies that are not available on the market,” Dr. Franco-Barraza notes. The COMET™ platform allows users to build and customize antibody panels, using previously validated off-the-shelf antibodies. Such flexibility is particularly valuable in cancer research, where unique and specific antibodies are often required to study various cellular markers.
High throughput and automation
COMET™ is a fully-automated, high-throughput, hyperplex platform for spatial analysis of FFPE and frozen tissues. It performs seqIF™ assays through staining, imaging, and elution cycles. With a 4-slide capacity, it processes 20 slides of 20-plex protein weekly and executes runs of 40-plex without human intervention.
This high-throughput capability is crucial for large-scale studies and clinical trials, where analyzing numerous samples consistently is essential.
Precision and detail
The platform’s ability to provide detailed spatial information at the single-cell level is another significant advantage. Dr. Franco-Barraza explains, “With COMET™ technology, we can now scrutinize every single cell within a tumor mass, studying not only cancer cells but also the interactions among various participants in the tumor microenvironment.” This precision allows researchers to assign scores to individual cells based on the expression of multiple biomarkers, offering insights into the functional status of the TME and its response to treatments.
Applications in pancreatic cancer research
Dr. Franco-Barraza’s research has primarily focused on ductal adenocarcinoma (PDAC), a particularly challenging type of cancer due to its dense and often immunosuppressive stroma. The Harmonized Output of Stromal Traits (HOST) Factor, developed by his team, integrates a proprietary biomarker dataset to measure the functional status of stromal cells, offering insights into tumor-promoting and tumor-suppressing phenotypes.
“Understanding key signalling pathways used by stromal and immune cells within the TME context offers many opportunities for therapeutic intervention,” Dr. Franco-Barraza explains. By utilizing COMET™, his team can analyze the composition and dynamics of the TME before and after treatment, helping to identify potential biomarkers for therapeutic response and disease progression.
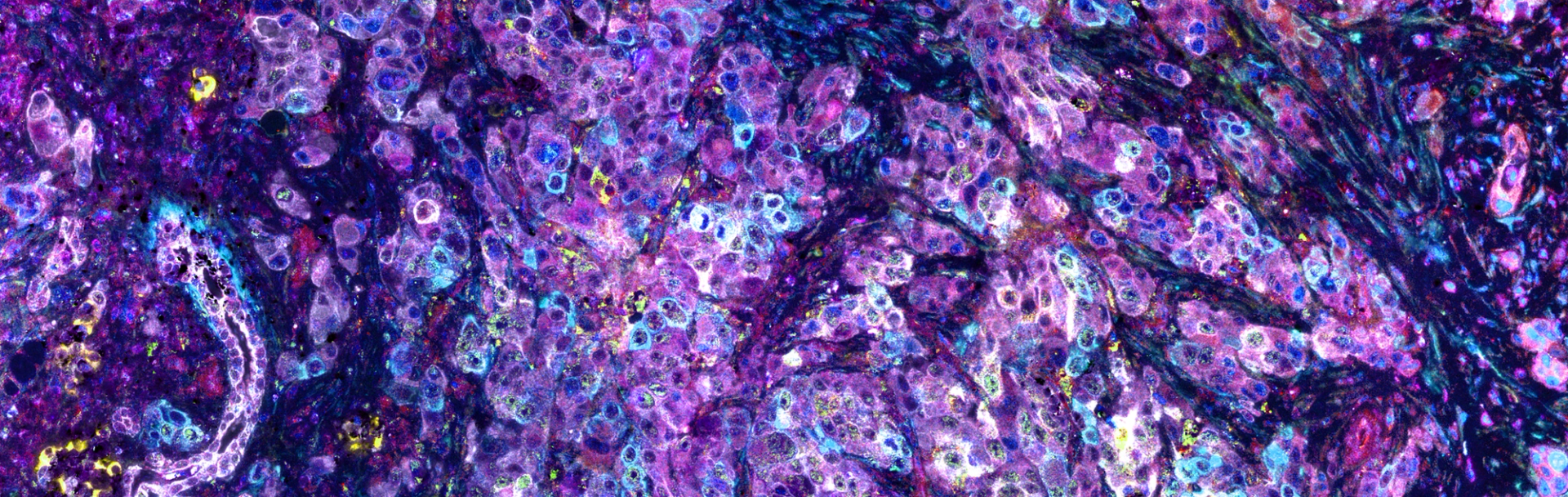
A section of pancreatic cancer tissue, highlighting cancer cells interspersed with stromal components, including fibroblasts and immune cells (resolution of 230 nm/pixel). These diverse cell populations were labeled with eleven distinct signals using seqIF™ technology, where each color corresponds to a different marker, illustrating the cells' functional states within the complex tumor microenvironment. Image was provided by Dr. Janusz Franco-Barraza and generated on COMET™.
Broader implications and future directions
While the initial focus has been on PDAC, the methodologies developed using COMET™ are adaptable to other types of cancer. Dr. Franco-Barraza emphasizes the importance of this adaptability, stating, “Studying the TME in PDAC not only enhances our understanding of this malignancy but may also reveal commonalities in the stromal characteristics of other solid cancers, expanding potential interventional options.”
Looking ahead, Dr. Franco-Barraza is excited about the potential to uncover new cell populations and their roles within the TME. “The ability to discover new populations of cells that haven’t been described before and to study them in detail is extremely exciting,” he says. This capability opens new avenues for understanding tumor biology and developing targeted therapies.
New heights in immuno-oncology research
Spatial biology is revolutionizing our understanding of the tumor microenvironment, offering new insights into cancer progression and treatment. The COMET™ platform stands out as a powerful tool in this field; its full automation, high throughput, and flexibility to work with off-the-shelf, non-conjugated primary antibodies make it an invaluable asset for researchers like Dr. Janusz Franco-Barraza, who are working to unravel the complexities of the TME and improve therapeutic outcomes for cancer patients. Learn more about the COMET™ platform and how it provides walk-away automation for staining, imaging, and image preprocessing, generating highly robust and reproducible data.

