How the pharma industry is harnessing cryo-electron microscopy in drug discovery
Learn how AstraZeneca is adopting nascent cryo-electron microscopy (cryo-EM) technology to expand its targets for drug discovery
4 Feb 2020
Structural biology is established within the heart of drug discovery, guiding an understanding of both disease mechanisms and how putative new drug compounds interact with protein targets. As a discipline, structural biology is highly technology-dependent and advances in knowledge of molecular structures have invariably been driven by advances in equipment. For the past 30 years, X-ray crystallography has been the workhorse technique, but cryo-EM has now emerged with the potential to transform drug discovery. Dr. Chris Phillips is a structural biologist and Associate Director at AstraZeneca in Cambridge, U.K., who leads a team harnessing cryo-EM for elucidating protein structure where conventional crystallographic approaches fall short. But just how might cryo-EM be a ‘game-changer’?
A new age of drug discovery?
Phillips is particularly excited about the potential of cryo-EM since it opens up new targets for structural biologists. It supports the whole of AstraZeneca’s drug development portfolio and has enabled the company to begin to look at membrane proteins and ion channels (as targets) which are often resistant to X-ray crystallography (figure 1). But it does not stop there. “Other types of proteins, such as multi-protein complexes, also would not have been considered by crystallography,” says Phillips.
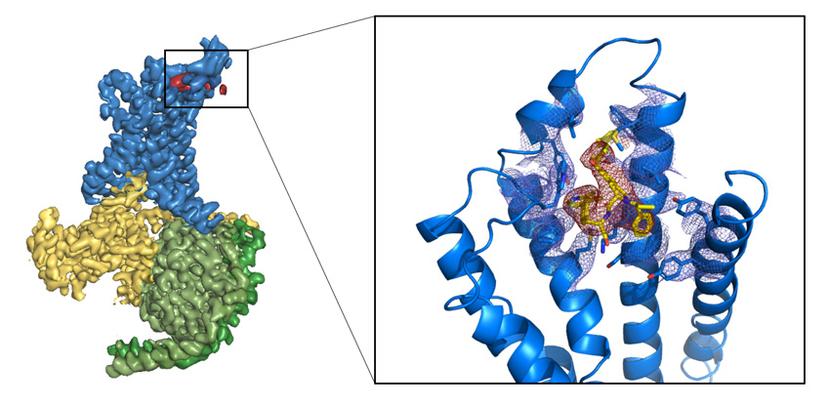
Whilst he suggests that it is too early to say whether or not cryo-EM has proved its worth, Phillips does not doubt its promise. “The GABA [gamma aminobutyric acid] structures emerging from the MRC’s Laboratory of Molecular Biology (LMB) in Cambridge are a fabulous example of pharmacology explained by cryo-EM,” he says.
Phillips adds that the greatest impact of cryo-EM so far within AstraZeneca has been in understanding more fully the mechanistic biology of the diseases in which the company is interested, such as cancer, diseases of the respiratory system, cardiovascular disease or neurodegenerative conditions. He points to a publication between AstraZeneca and University College London that has just been accepted on the cryo-EM structure of the enzyme phospholipase Cγ1 in complex with a kinase FGFR1, the former being a key signaling hub in resistance to some cancer drugs such as receptor tyrosine kinase (RTK) inhibitors and Bruton’s tyrosine kinase (BTK) inhibitors. The structure, even at a modest resolution, provides a mechanistic understanding of why mutations in the protein give rise to the resistance that is emerging, particularly to BTK inhibitors. “The information is novel. The phospholipase C enzyme has been studied structurally for a very long time – its individual domains have been classically solved by crystallography – but we have been able to use cryo-EM to show how they all come together in the complex,” says Phillips.
Access to cryo-EM does not come cheaply, however, so how has AstraZeneca managed to overcome this particular barrier?
The consortium approach to accessing cutting-edge cryo-EM
Phillips’s structural biology team has access to two state-of-the-art Thermo Fisher Scientific Krios cryo-transmission electron microscopes with fast cameras. These have been made available through the Cambridge Pharmaceutical EM Consortium, a collaboration between Thermo Fisher, the MRC’s LMB and five pharmaceutical companies from the Cambridge area – AstraZeneca, GlaxoSmithKline, UCB, Heptares and Astex. None of the five companies would likely have invested alone in cryo-EM at the outset in 2016, given the cost of the equipment and the time required to gain expertise in its use, but “the Cambridge-area cryo-EM community has been invaluable,” observes Phillips. On Thermo Fisher, he adds that “they are the market leaders in top-end microscopy – this is what everybody buys!”
The consortium has been a huge success, such that the companies involved already have, or are beginning to develop, their own in-house capabilities. Phillips asserts that this is a great time to be a young scientist in cryo-EM, noting that “it is ‘exploding’ as a structural biology technique and is having a huge impact across academia and now industry.”
Future developments
Cryo-EM is a nascent tool in the drug discovery industry, though it has the potential to become a frequently used technology in the early stage of the drug discovery paradigm (target selection and lead discovery) alongside existing methods such as X-ray crystallography and nuclear magnetic resonance (NMR).
Phillips is already clear as to how he would like to see the technique develop in the future, in the form of a more ‘industrialized’ process. “It needs to deliver high-resolution structures more routinely and much faster. The technology that allows us to do that is key, such as better cameras and a better understanding of how to prepare samples,” he says.
It all leaves little doubt that cryo-EM has the potential to expand the pharmaceutical industry’s pursuit of more classes of protein targets beyond that currently possible with other techniques.
References
Zhao, P., Liang, Y., Belousoff, M.J. et al. Activation of the GLP-1 receptor by a non-peptidic agonist. Nature (2020) doi:10.1038/s41586-019-1902-z
Want to know more?
- Download the application eBook: Structure-based drug discovery with cryo-EM
- Read how Dr. Claudio Ciferri, structural biologist, crystallographer and now head of cryo-EM at Genentech, uses cryo-EM in the investigation of the structure and function of challenging protein targets
- Find out more about Thermo Fisher Scientific's cryo-EM tools for drug discovery


