How to optimize your cell-based assays: Overcoming common challenges
Cell culture expert Ann-Cathrin Volz discusses current trends and challenges in the field and shares a solution
25 Oct 2021

As a versatile technique, cell-based assays are used across a range of scientific areas, from drug discovery and screening to vaccine development and SARS-CoV-2 research. In this SelectScience® interview, Ann-Cathrin Volz, applications specialist at BMG LABTECH, explores the latest developments in the area as well as common hurdles faced by scientists, and offers expert insight to help solve these issues and enhance results.
Tell us a little about your role at BMG Labtech
ACV: I have been part of the BMG LABTECH team as an applications specialist for more than one year now. Before I started to work for BMG LABTECH, I myself have benefited several times from the company's excellent support — it's great to be a part of it now.
I gather information on the latest trends and make it available to our customers in the form of application notes, blogs, articles, webinars and other means so that they can quickly get an overview of what's going on. Part of my work is also to practically implement and optimize emerging applications to support our customers in their research projects. Ongoing support during troubleshooting is of course also part of this.
Within the Applications Team, my position focuses specifically on cell-based assays. Here, I can draw on my many years of experience in cell culture and tissue engineering.
What are some of the current trends in cell-based assays?
ACV: In drug discovery, cell-based assays offer consistent tissue-specific responses in biologically relevant microenvironments, in contrast to biochemical assays. Therefore, they have become a key component in the drug development process to study cytotoxicity, biological activity, biomechanical mechanisms and off-target interaction in the lead identification and optimization processes. Cell-based assays thereby help to ensure greater efficiency in the drug discovery and development process.
Traditionally, cell-based assays have been evaluated with endpoint measurements based on cell lysates or similar preparations. In contrast, live-cell assays represent a more sophisticated approach, since they enable real-time measurement of processes that are difficult to capture by destructive methods. Such assays preserve structure and physiological context and can be used to quantitatively measure cellular behavior over time to understand the influence of environmental cues. As probes and methodologies for the analysis of living cells improve, live-cell assays are becoming more popular.
Finally, cell-based assays are a versatile tool in virology as recently shown in SARS-CoV-2-related research. They are used in viral infection research, in vaccine development and drug screening. It is therefore not surprising that cell-based virological methods have been also extensively used during the COVID-19 pandemic. Among them, the most popular approach is the viral infection assay, whereby the proportion of virus-infected cells is determined either by monitoring cytotoxicity of the host cells or by fluorescent/luminescent reporters expressed by the viruses. This assay can be applied to screen for antiviral drugs or generally study virus-host cell interaction.
What challenges do scientists face when running these assays and how can a microplate reader help them?
ACV: The application of advanced cell-based assays in the early phase of drug discovery results in the need for upscaling and transfer to high-throughput handling. The cost of using cell-based assays can only be kept in balance if the required volumes are greatly reduced and the speed of measurement is significantly increased. Being able to measure with high sensitivity up to thousands of samples in one plate with nanolitre volumes, microplate readers allow for all manner of reproducible, cell-based assays to be done in just minutes. Thus, microplate readers provide a holistic assistant in the transition of assays into high-throughput procedures.
The superiority of modern cell systems results from the use of specialized cell types as well as co-cultures and 3D cultures. However, these sophisticated systems come with special atmospheric requirements: cells are often sensitive to changes in ambient temperature, CO2 as well as O2 levels, especially in long-term kinetic experiments. In addition, the establishment of physiological systems often requires special conditions, such as a hypoxic environment. Microplate readers offer an option to create a cell-friendly environment. With its Atmospheric Control Unit, BMG LABTECH enables control of temperature, CO2, and O2 and even allows gas ramping.
Another challenge can be the complexity of cellular systems and the required readouts. In contrast to biochemical approaches, which mostly consist of a homogeneous liquid sample, adherent cells are mostly unevenly distributed across the bottom of the well. Their evaluation is therefore strongly dependent on the measuring position. Features like well scanning or orbital averaging compensate for this heterogeneity, as they cover the well bottom with multiple measurements and thereby average local inequalities.
Finally, in contrast to classic biochemical approaches, cell-based assays can be used to assess multiple readouts simultaneously. These are necessary to be able to draw conclusions on the interdependent and complex physiological data. Multi-mode microplate readers facilitate multiplexing, allowing different assays to be performed simultaneously in a single measurement. This frees space and time which can be used to analyze additional samples, thereby increasing throughput even further, and reduces resource expenditure as you can simply get more information out of your sample.
What are the key considerations to help optimize cell-based assays results when using a microplate reader?
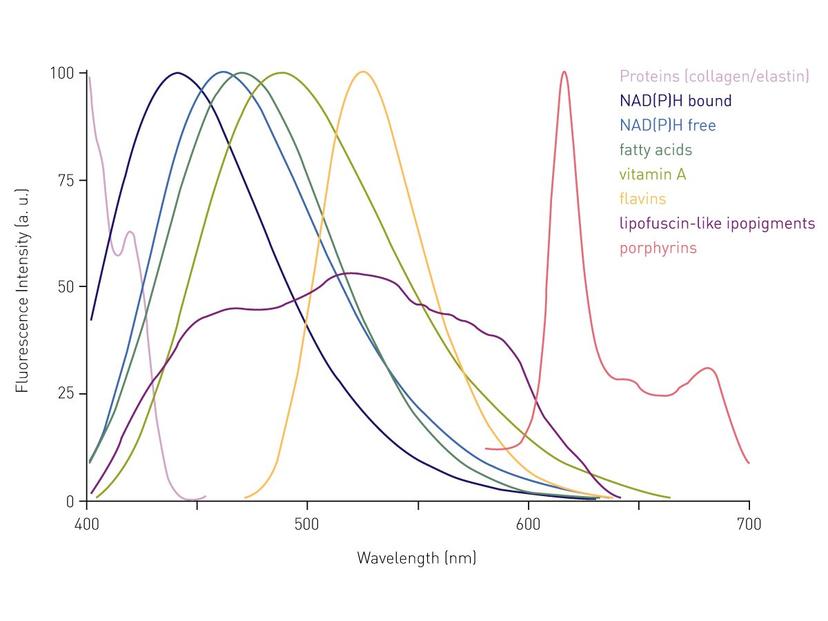
ACV: Try to avoid autofluorescence! Cellular components as well as media components such as phenol red exhibit high autofluorescence, which can significantly affect the readout. Generally, for fluorescent applications, the use of phenol red-free media is highly recommended to reduce the background. The autofluorescence derived from cellular components mainly takes place in the green range and can only be excluded using red-shifted dyes (> 570 nm). Dedicated red-shifted detectors measure the fluorescence sensitively up to 900 nm and, in contrast to standard photomultiplier tubes, thereby also cover (infra)red dyes. Since the signal in adherent cell cultures is mostly concentrated on the bottom of the well, it is recommended to perform the data acquisition from below the plate instead of the top. Thereby, the light does not have to pass the cell culture supernatant above the cells.
Choose your microplate type wisely. Bottom reading can obviously not be done with standard plates, microplates with a clear bottom have to be used here. Quartz and glass bottom usually perform better than plastic but are also significantly more expensive and more difficult to handle. Depending on the assay, the walls of the wells should be either transparent (absorbance), white (luminescence) or black (fluorescence). Plate selection primarily affects very low signals and those close to the upper detection limit, as plate color influences background noise and signal gain. Black plates with a clear bottom are most used for cell-based assays since they minimize background and inhibit crosstalk.
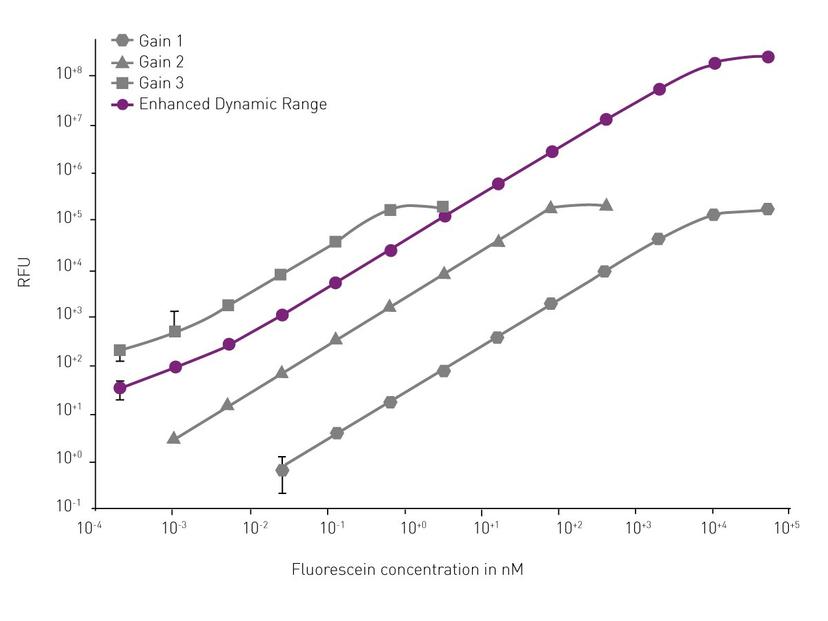
Finally, keep your cell density in mind. The lower limit of detection depends strongly on the cell type and the dye used. We recommend playing around with the cell density in preliminary experiments to identify the best possible dynamic window of the respective assay. Even if the cell density is optimally set at the beginning of the assay, the signal development over time can often not be predicted especially for long-term kinetic measurements over several days. Very dim signals are usually measured with a high gain, to amplify the signal and ease its detection. Very bright signals are usually measured with a low gain to keep them in the detectable range. If signals vary strongly, or increase/decrease extremely over time, gain setting is often a compromise. Sometimes the results are completely unusable due to an improper setting. The Enhanced Dynamic Range (EDR) feature from BMG LABTECH provides the largest possible dynamic window (eight concentration decades) making manual gain adjustments superfluous and eliminating the risk of detector saturation.
What do you see for the future of cell-based assays and the technology that supports them?
ACV: Researchers keep focusing their attention on more complex questions and heterogeneous diseases. Therefore, more complex, and biologically relevant assays must be developed to address these queries. The CRISPR Cas9 technology has revolutionized the study of genes and their functions as it allows very accurate and easy genome editing. CRISPR greatly simplifies the development of more complex disease-relevant cell-based assays. Going more physiological also includes the switch from immortalized cell lines to differentiated, fully functional primary cells of human origin. The detection of endogenously expressed target molecules on a physiological and therefore smaller scale is also becoming increasingly important.
The more physiologic and complex the models get, the more complex and subtle the possible readouts will be, therefore highly sensitive instruments are needed to provide robust data. Modern microplate reader technology is capable of measuring very low signals with high sensitivity and is therefore well prepared to follow this development.
