Increasing SARS-CoV-2 testing capacity with CRISPR-based rapid testing
Genome editing experts harness the power of CRISPR-Cas enzymes to power the next diagnostic revolution in COVID-19 detection and diagnosis
16 May 2021
The COVID-19 pandemic has not only changed the way the world views communicable diseases but has also placed significant strain on the diagnostic testing services that are struggling to keep pace with current needs. To help actively curb COVID-19 spread, it is critical that testing laboratories deliver short turnaround times for effective management of the disease. While the gold standard in COVID-19 testing remains time-consuming PCR testing, lateral flow antigen tests provide a rapid, effective, and inexpensive option. However, there is a clear need to bridge the gap between the two methods and increase molecular testing capabilities through simple, rapid, and programmable solutions.
As experts in CRISPR-Cas enzymes and their functioning, California-based biotech startup Mammoth Biosciences is at the forefront of this evolution in diagnostics. Through the discovery and harnessing of novel CRISPR systems, the company is powering the next generation of CRIPSR products to expand its future applications. Providing an insight into Mammoth’s vision, Tim Patno, Vice President of Product Development at Mammoth Biosciences, says: “Mammoth’s objective is to harness CRISPR technology to read and write the code of life. We ‘read’ by using CRISPR-Cas enzymes to detect sequences of RNA or DNA, for example, to diagnose diseases. We ‘write’ by utilizing the same enzymes to add sequences into genomes that will repair and fix debilitating diseases.” In this SelectScience® article, Patno discusses this potentially game-changing technology and reveals how collaboration with industry leaders has propelled Mammoth Biosciences towards greater success.
An efficient and accessible testing solution that overcomes supply chain challenges
A revolutionary method for molecular diagnosis, the Mammoth Biosciences CRISPR-based platform has significant potential due to its speed and simplicity, making it especially valuable during surges in demand for diagnostic services, as seen in the COVID-19 pandemic. For this purpose, Mammoth’s propriety DETECTR BOOST™ SARS-CoV-2 Reagent Kit, a high-throughput COVID-19 test targeting around 1,500 tests every 8-hour shift, is a good example of how CRISPR nucleases can be harnessed to make diagnostics accessible.
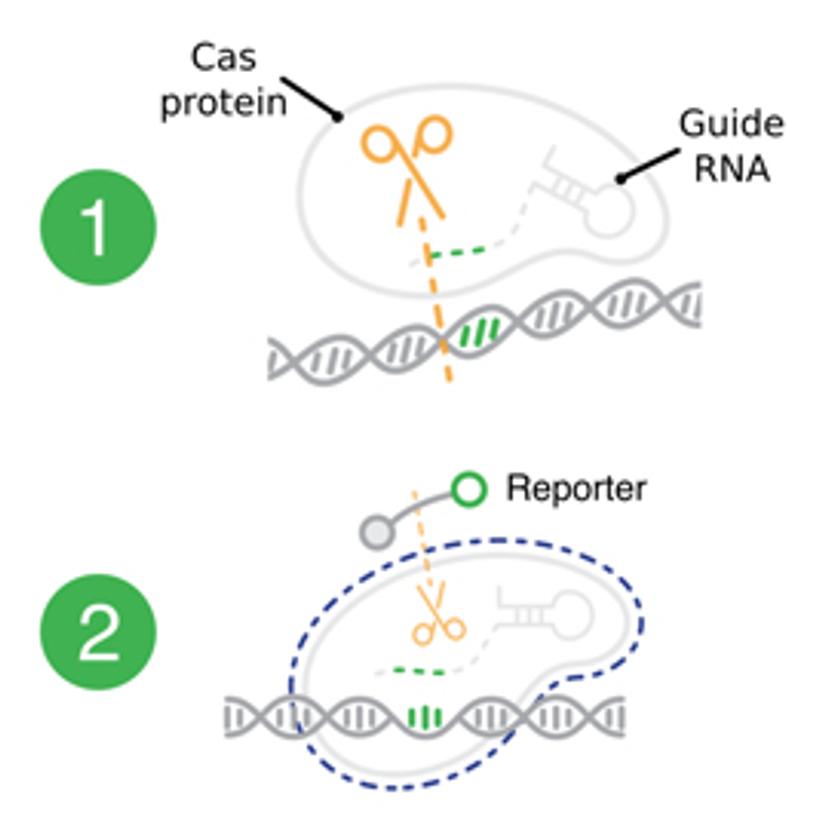
As the demand for increased testing capabilities peaks with increases in COVID-19 cases, matching the pace of the pandemic is vital. However, this is hindered by disruptions in equipment, reagent and consumable supply chains, shortages in technical laboratory skills and the throughput capabilities of current platforms. The DETECTR BOOST™ platform aims to provide a new solution that can be run on widely pre-existing liquid handling instruments in laboratories and allows for rapid sample processing in an almost fully automated setting. “CRISPR-Cas enzymes are unique as they enable a different distribution chain employing an instrument-agnostic and disposable format. Using these enzymes could enable us to work around many of the supply chain challenges that we are currently facing during this pandemic,” explains Patno.
With the launch of the National Institute of Health’s prestigious Rapid Acceleration of Diagnostics (RADx) program, Mammoth has recently received funding to scale its CRISPR-based testing workflow, testifying to its potential to transform the biotech supply chain. However, the journey ahead for diagnostic visionaries such as Mammoth is one fraught with scientific and technical difficulties cautions Patno, with the pandemic resulting in intermittent access to different formulation components and even consumables such as pipette tips. Patno describes how collaborations with industry leaders have been instrumental in dealing with these challenges: “All of the companies involved in diagnostics have been affected by the pandemic. Many different components have been difficult to get, and Merck has been a great ally in helping us obtain components that a small company like Mammoth would otherwise struggle with.”
To deal with the surges in demand in the face of consumable shortages, Mammoth focused not only on supply, but also on consciously designing workflows that use fewer consumables. Typically, an assay uses over 10 tips to go from sample to result. The team at Mammoth has worked to get that number down to six or seven tips, creating a lean and efficient reagent kit in the DETECTR BOOST™ platform. It then further developed the optimized product formula with Merck, who is currently helping to replicate and mass-produce the kits.
Collaboration for successful product scale-up and positive impact
For the successful production and commercialization of a product, technical expertise, scale-up, as well as proven manufacturing knowledge are all equally critical, explains Patno. “Formulating reagents in large-scale manufacturing volumes is vastly more difficult than people imagine it to be. In research or early development settings, errors in the formulation steps can be easily rectified by simply re-doing them. In manufacturing, it is essential to be right because, in high-volume production, mistakes can potentially end up costing hundreds of thousands of dollars. When we were scouting for partners to help us in scaling up our custom reagents, Merck stood out as a contract manufacturing leader that knew what it was doing and knew how to do it well.”
Merck stood out as a contract manufacturing leader that knew what it was doing and knew how to do it well.
Tim Patno Mammoth Biosciences, Inc.
Patno asserts that key to the success of the Mammoth Biosciences-Merck partnership is the combination of adaptability and speed that are hallmarks of both companies. Having jointly manufactured multiple batches of CRISPR-Cas enzymes and other reagents, Patno believes that this collaboration is also strengthened by a shared outlook that is primarily vision-driven: “The people we work with daily at Merck find the most flexible and efficient way to get tasks done within their available choices," notes Patno. "They are highly capable, but they are more than just that; they are truly passionate about wanting to help in this pandemic. I have worked in molecular diagnostics for many years. I have seen alliances where companies just want to know what their margin is. But, through their words and actions, Merck has shown us that they truly believe that they succeed when Mammoth succeeds.”
Getting future ready: Decentralizing diagnostics
Having witnessed and contributed to the lab automation revolution that transformed the use of molecular diagnostics, Patno predicts that the next revolution will involve making diagnostics readily available outside of the lab. And this pandemic has provided clear evidence that when tackling an infectious disease, it can be detrimental to depend on purely lab-based tests with more than a day’s turnaround. The natural next step is to optimize and incorporate rapid diagnostic testing at point-of-care settings such as pharmacies, schools, occupational work offices and eventually into our homes. “At Mammoth, we strongly believe that the CRISPR-Cas enzymes that we are developing and making available are going to be key components of decentralizing the future of rapid molecular diagnostics,” concludes Patno.
For more information on increasing molecular testing capabilities, see the related resources below.
Video: Bringing revolutionary CRISPR-based diagnostics to market.
In this video, Dr. Yves Dubaquie, Head of Diagnostics Solutions at Merck, joins Tim Patno to describe how collaboration with industry leaders can supply the manufacturing expertise and quality critical raw materials needed for success.
Application notes:
The Life Science business of Merck operates as MilliporeSigma in the U.S. and Canada.