MEET and Shimadzu revolutionize battery research
Dr. Sascha Nowak from MEET Battery Research Center showcases their research collaboration with Shimadzu, focusing on electrolyte aging, metal migration, recycling, and battery safety using advanced instruments for sustainable battery technologies.
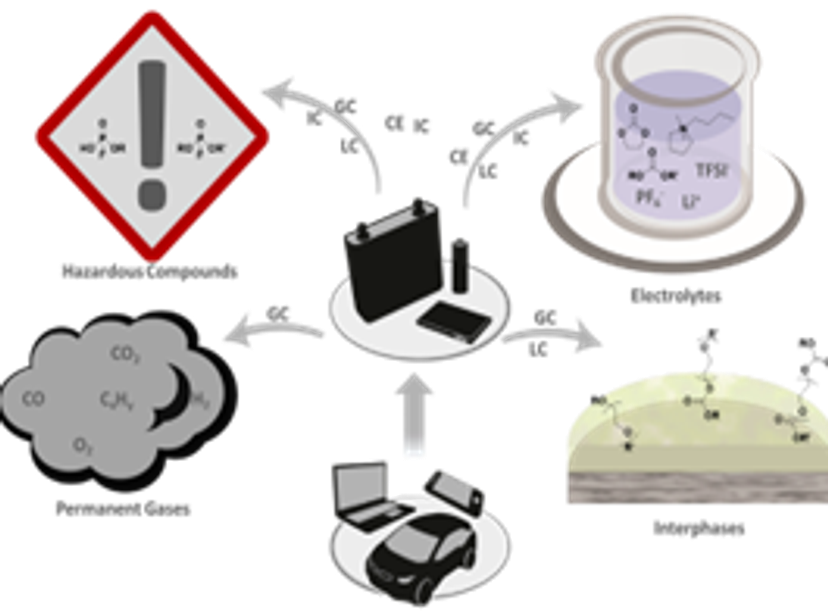
5 Jul 2024At the MEET Battery Research Center, pioneering research is underway under the leadership of Dr. Sascha Nowak, Head of the Analytics & Environment Division. Recently partnering with Shimadzu, a leading analytical instrument manufacturer, MEET, located at the University of Münster, is spearheading Germany's advancements in battery research. Their focus spans across electrolyte aging, metal migration, recycling, and battery safety, utilizing cutting-edge methodologies supported by Shimadzu instruments. Dr. Nowak sheds light on their impactful discoveries and the instrumental role of Shimadzu in shaping the future of sustainable battery technologies and recycling practices.

Dr. Sascha Nowak, head of the division Analytics & Environment of the MEET battery research center in Münster, Germany.
Q: Could you outline the research and let us know what discovery and achievement have been made so far?
SN: We focus on (electrolyte) aging, transition metal migration and surface investigations, recycling and second life as well as toxicological aspects with regard to working safety. With many newly developed methods, we could discover and verify aging products and mechanisms. Furthermore, all applied methods were transferred to the recycling of lithium-ion batteries to ensure the identification of the recycling components and quality control during the recycling processes.
Q: Could you introduce the research that has been particularly successful among your many advanced researches?
SN: The competence field electrolyte aging characterizes electrolytes in their various states of operation. Aging effects are observed in terms of qualitative and quantitative investigation using different analytical methods. This also includes the synthesis of substances as standards that are not commercially available. In addition, recycling methods like extracting the electrolytes from cells are analytically developed and validated.
Supercritical gases with and without the addition of solvents are being tested for the extraction of electrolyte components from spend batteries. The extraction is carried out either in an autoclave or in commercially available extraction units. To characterize the electrolyte, a wide variety of methods that focus on identification and quantification of the different degradation compounds are used. Reaction schemes are concluded from these measurements that provide a deeper understanding of the manifold processes taking place inside a battery.
Furthermore, migration of metals into the electrolyte and deposition on the electrodes is investigated. Here, the focus is not only to determine total amounts, but also to examine species of migrated metals and the factors influencing this process. This helps to combine electrolyte degradation mechanisms with the aging processes of electrodes.
The methods used in electrolyte aging to identify and quantify the aging/decomposition products are also applied to evaluate and determine the risk given by hazardous substances in cooperation with toxicologists. This also includes an overall evaluation of the electrolyte to meet safety criteria. Another focus of toxicological examination is the investigation of degradation products that may be formed during/origin from the extraction, processing and further storage of recycled material. These are analyzed for their possible toxicity. The results are then included in an evaluation of the entire recycling process and of the individual steps in this process with regard to their specific potential risk.
Investigation of electrolyte degradation products.
Q: How are Shimadzu instruments helping in your research?
SN: We are mainly using GC instruments with different detectors (TCD, BID, MS and FID) and sampling possibilities like HS and SPME [cf. Leißing et al, (2020)]. The analysis of the electrolyte is the focus with regard to the aging mechanisms. Additionally, we implemented a pyrolysis system to investigate high boiling compounds like binder and separators [cf. Stenzel et al, (2019)]. As a second step, we added a LCMS-IT-TOF™ to our portfolio, which further helped us broadening our application range [cf. Henschel et al, (2019a)].
Q: Why did you chose Shimadzu as your partner when you started this project?
SN: We got some recommendations and the possibility to use a fully equipped robotic autosampler on an GC instrument having different detectors installed in one setup was helpful in the beginning of our research. For example, we had the opportunity to include several detectors like FID and MS with a two-column setup, AOC-5000 autosampler and an optic injector as additional inlet system in one GC setup.
Furthermore, the robustness of the systems was quite impressive with regard to our application, since we handle quite complex and difficult samples, including a highly concentrated fluorinated salt, which can release HF and other fluorinated compounds.
Q: What are Shimadzu’s strengths compared to other vendors?
SN: Since the application was quite new, even unusual requests with regard to instrumental setups could be discussed and realized which really helped us to advance further with our methods and understanding.
Q: Finally, could you share any requests that you have with respect to analytical and measuring instrument vendors?
SN: In general, quantitative information is always needed. However, since standard and reference materials are not available so far, speciation is the method of choice now. Therefore, further advancements in this area, whether automated coupling or robust and interference-free detection are welcome.
Recent publications using Shimadzu instruments
Leißing M, Winter M, Wiemers-Meyer S, Nowak S. 2020. ‘A Method for Quantitative Analysis of Gases Evolving During Formation Applied on LiNi0.6Mn0.2Co0.2O2 II Natural Graphite Lithium Ion Battery Cells Using Gas Chromatography - Barrier Discharge Ionization Detector.’ Journal of Chromatography A 1622: 461122. doi: 10.1016/j.chroma.2020.461122.
Stenzel YP, Börner M, Preibisch Y, Winter M, Nowak S. 2019. ‘Thermal profiling of lithium ion battery electrodes at different states of charge and aging conditions.’ Journal of Power Sources 433: 226709. doi: 10.1016/j. jpowsour.2019.226709.
Henschel J, Schwarz L, Glorius F, Winter M, Nowak S. 2019a. ‘Further insights into structural diversity of phosphorus-based decomposition products in lithium ion battery electrolytes via liquid chromatographic techniques hyphenated to ion trap - time of flight mass spectrometry.’ Analytical Chemistry 91, Nr. 6: 3980-3988. doi: 10.1021/acs.analchem.8b05229.
