Knockout validation: Confirming antibody specificity
3 May 2020
Knockout (KO) validation via CRISPR-Cas9 gene editing delivers the highest level of confidence in antibody specificity to life science researchers. Abcam is adopting this technique to ensure its antibodies provide the required level of specificity to drive research reliability and translation.
The need
Antibodies are amongst the most commonly used tools in life science and clinical research for the study of proteins and their functions within various biological pathways and diseases.
A good antibody exhibits target specificity and sensitivity, allowing it to identify the protein of interest at even at low expression levels. However, an increasing number of studies have shown that not all antibodies are specific in this way, with many showing cross-reactivities with off-target proteins. This has led to the recognized issue of experimental irreproducibility. These non-specific antibodies result in wasted resources and compromise the advancement of science.
The solution: Knockout validation
One of the most accepted and trusted validation processes for antibody specificity is knockout (KO) validation. KO validation is an incredibly robust technique used to confirm antibody specificity by testing the antibody of interest in a KO cell line or tissue that does not express the target protein.
A specific antibody should yield no signal when tested in a KO cell line data while giving the specific target protein signal in the wild-type (WT) cell line. In this way, KO validation serves as a true negative control to confirm antibody specificity to the protein of interest.
Abcam use an extensive library of human KO haploid cell lines to perform antibody validation. These KO cell lines are generated via CRISPR-Cas9 and certified via Sanger sequencing. This type of KO cell line provides a complete loss-of-function phenotype from a single allele KO and eliminates any masking of the knockout from a second allele seen in diploid cell models.
Knockout validation testing results
When KO-validating its antibodies, Abcam primarily use western blots to assess the results. A range of western blot outcomes can result from KO validation, and the following examples demonstrate how we deal with each situation at Abcam.
1. Specific antibody
- Result: The expected molecular weight (MW) band disappears in the KO sample.
- Next steps: KO-based specific data added to the datasheet. Antibody guaranteed for specificity via KO testing.
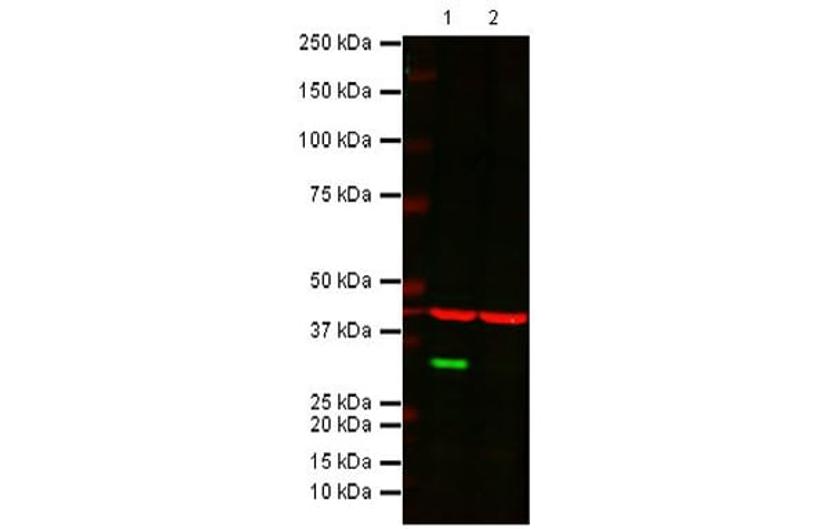
The expected band at the correct MW disappears in the KO sample. All lanes: Anti-Cdk4 rabbit monoclonal antibody [EPR4513] (ab108357) at 1/1000 dilution. Lane 1: Wild-type HAP1 cell lysate. Lane 2: Cdk4 Knockout HAP1 cell lysate. Predicted band size: 34 kDa. Observed band size: 34 kDa. Loading control (red band): Anti-beta Actin antibody (ab8226) at 1/1000 dilution. Secondary antibodies: IR Dye 800 Goat anti-Rabbit IgG (H + L); IR Dye 680 Goat anti-Mouse IgG (H + L), both at 1/10,000 dilution.
2. Partially specific antibody – extra bands
- Result: The expected MW band disappears in KO, but extra bands are present at a distinct MW.
- Next steps:Abcam adds KO-based specific data to the datasheet. Additional research and testing will be performed to identify the extra bands.
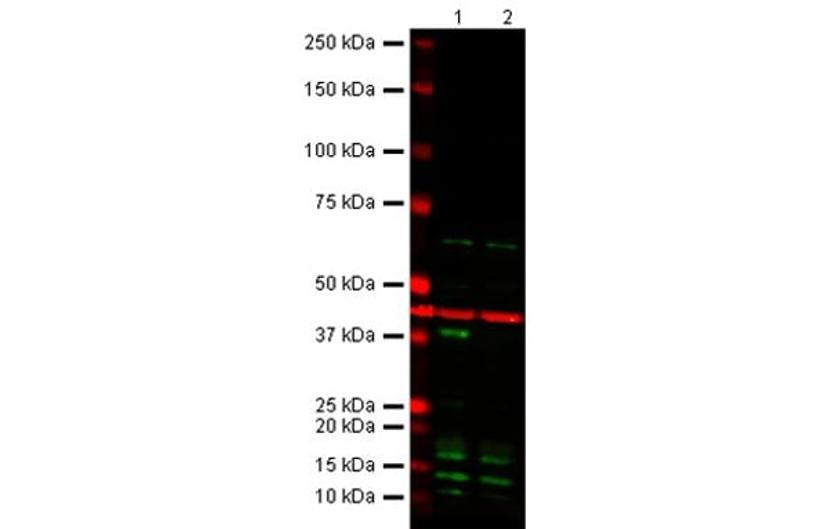
Figure 2. Cdk6 Mouse monoclonal product (ab54576).
The expected band at the correct MW disappears in the KO sample; thus, the antibody recognizes its intended target. However, extra bands are also present in both WT and KO samples. This may be due to antibody cross-reactivity with other protein family members/isoforms or unrelated proteins. All lanes: Anti-Cdk6 antibody (ab54576) at 1/1000 dilution. Lane 1: Wild-type HAP1 cell lysate. Lane 2: Cdk6 Knockout HAP1 cell lysate. Predicted band size: 37 kDa. Observed band size: 37 kDa. Loading control (red band): Anti-beta Actin antibody (ab8227) at 1/1000 dilution. Secondary antibodies: IR Dye 800 Goat anti-Mouse IgG (H + L); IR Dye 680 Goat anti-Rabbit IgG (H + L), both at 1/10,000 dilution.
3. Non-specific antibody – weak signal in the KO sample
- Result:The expected MW band still present in KO at a lower intensity compared to WT. A benchmark antibody resolves a target band in WT and no band in KO.
- Next steps:Abcam adds KO-based specific data to the datasheet. Additional research and testing will be performed to identify the reasons behind the signal in the KO sample.
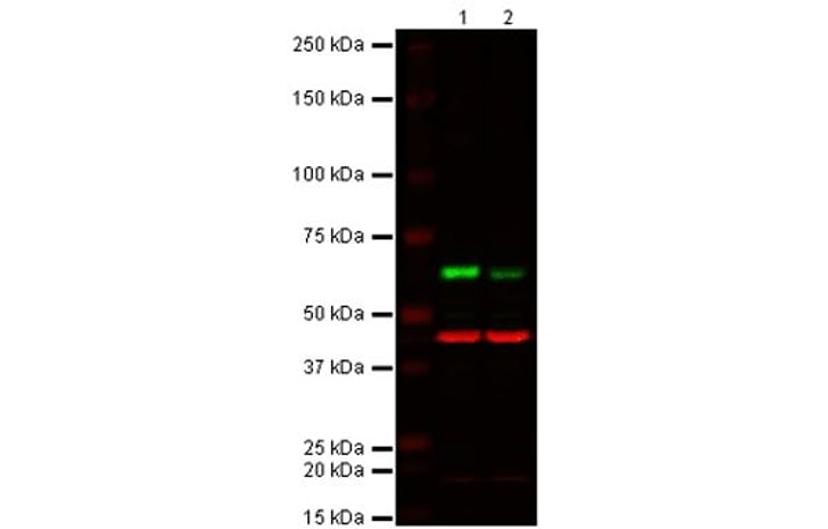
The expected band at the correct MW is present in the KO sample but at a lower intensity compared to WT sample. However, the antibody may be exhibiting cross-reactivity with other protein family members/isoforms which are present at the same molecular weight in the sample as the target protein. All lanes: Anti-Akt1 rabbit monoclonal antibody (ab32038) at 1/1000 dilution. Lane 1: Wild-type HAP1 cell lysate. Lane 2: Akt1 Knockout HAP1 cell lysate. Predicted band size: 55 kDa. Observed band size: 55 - 60 kDa. Loading control (red band): Anti-beta Actin antibody (ab8226) at 1/1000 dilution. Secondary antibodies: IR Dye 800 Goat anti-Rabbit IgG (H + L); IR Dye 680 Goat anti-Mouse IgG (H + L), both at 1/10,000 dilution.
4. Non-specific antibody – signal in the KO sample
- Result: expected MW band still present in KO at the same intensity as WT. A bench-mark antibody resolves a target band in WT and no band in KO.
- Next steps: Abcam removes the antibody from the catalog, and we recommend an alternative antibody with confirmed target specificity.
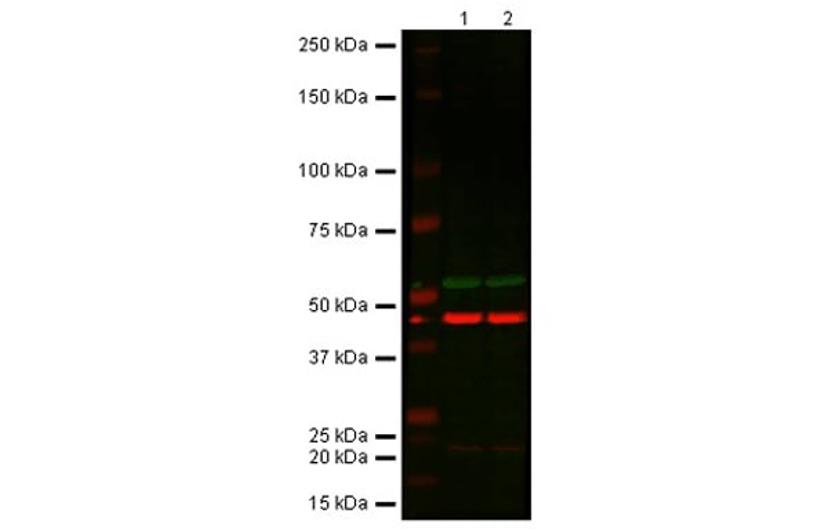
The expected MW band is still present in the KO at the same intensity as the WT. Use of a bench-mark antibody shows a target band in the WT and no band in KO samples. All lanes: Anti-Akt1 rabbit polyclonal antibody (ab91505) at 1:1000. Lane 1: Wild-type HAP1 cell lysate. Lane 2: Akt1 Knockout HAP1 cell lysate. Predicted band size: 55 kDa. Observed band size: 55 - 60 kDa. Loading control (red band): Anti-beta Actin antibody (ab8226) at 1/1000 dilution. Secondary antibodies: IR Dye 800 Goat anti-Rabbit IgG (H + L); IR Dye 680 Goat anti-Mouse IgG (H + L), both at 1/10,000 dilution.
5. Non-specific antibody – no target band in both KO and WT samples
- Result: The antibody does not recognize the target band in both the WT and KO samples. A bench-mark antibody resolves a target band in WT and no band in KO.
- Next steps: Abcam removes the antibody from the catalog, and recommend an alternative antibody with confirmed specificity.
Knockout-validated antibodies: Abcam's promise
When you see the KO-validated seal, you can trust that the antibody has not only been validated in the recommended applications and species, but its specificity has been confirmed through our in-house KO validation approach.
See Abcam's complete list of KO-validated antibodies>>
Knockout cell lines and lysates: Edited and ready for you
Application of CRISPR-Cas9 technology has allowed Abcam to develop an extensive range of KO cell lines and lysates, so that it can provide you with reliable antibodies, saving you time and money. Each KO cell line is individually cloned, validated by Sanger sequencing, and supplied with the parental WT control lysate to allow the biological impact of the knockout to be assessed within a consistent cellular background.
Abcam believes in being transparent with its customers regarding the performance of its antibodies and work with customers to offer the best reagents that are extensively validated to ensure the longevity of their research. While many researchers have adopted the use of monoclonal antibodies for increased target specificity, transitioning to KO-validated antibodies offers the gold standard for reliability in the results for the life sciences field. The additional assurance provided by KO-validated antibodies could aid in the translation of research discoveries to diagnostic or drug development pipelines, offering important validation evidence for tools and targets.
Want more of the latest science news straight to your inbox? Become a SelectScience member for free today>>
