Laboratory Water Essentials: Top Tips for Safeguarding Your Experiments
27 Oct 2014
When it comes to bench-work, detail is the name of the game: we do what we can to try to control every conceivable variable, but some things are just out of our hands. Is it an unusually hot summer’s day outside? Well, your qPCR or protein stability results could end up looking a little strange. Is your lab unfortunately positioned below the geology department and their crazy rock-shaking devices, or even near a railway? Then it could well mean stray data points in your delicate physics experiments. Or you may even find that the lab assistant with a cold has very effectively contaminated all of those culture plates you just poured!
However, in spite of meteorological phenomena or unpredictable cases of infected lab staff, there are important factors that we can, and should, control. One vital and often-overlooked variable is the water you’re using.
You use humble H2O in everything from cleaning benches and feeding autoclaves to preparing buffers and diluting samples for analysis, but it may not be something you consider when planning your experiments. To help, we’ve compiled some quick insights that we hope will ensure your experiments produce the data you expect, simply by fully understanding the water you use.
1. Organic and inorganic contaminants
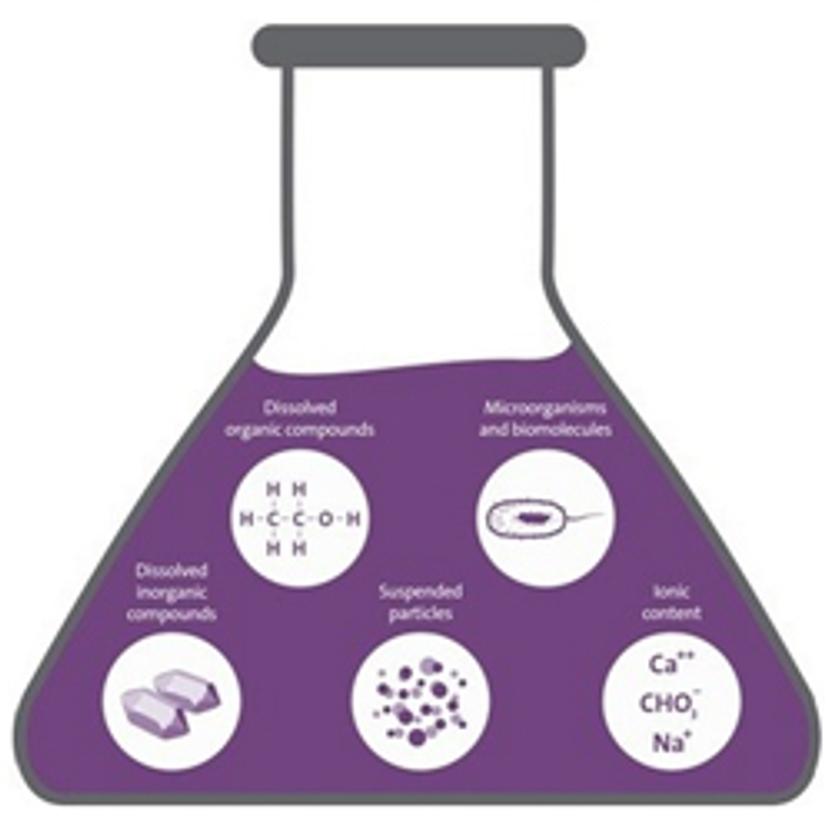
There is a huge number of possible water contaminants that can result in skewed experimental results, but for convenience, we’ll group them into inorganics, organics and suspended particles.
Dissolved inorganic compounds, which include the likes of sodium, iron, calcium, nitrates and sulfates, tend to make up the bulk of possible impurities lurking in your lab water. Left unchecked, these inorganics can affect experiments involving proteins, e.g. by interfering with protein-protein or protein-lipid interactions. Common cations such a Cu2+ , Fe2+ and Ni2+ can also easily upset enzymes such as DNA polymerase, disrupting substrate binding or even inhibiting activity entirely, meaning your PCR results could end up looking a little sparse (also known as the ‘curse of the empty gel’).
You also need to keep dissolved organics in check; a build-up of organic compounds is essentially like leaving a “Bacteria eat for free!” sign outside (we’ll look at bacteria in more detail shortly).
Certain organic compounds can be somewhat attention-craving: they’ll vie for center stage during your high performance liquid chromatography (HPLC) experiments, competing with the analyte in the mobile phase. They even want to get involved with your blotting experiments, non-specifically binding in place of RNA or DNA during the hybridization process and then leading to some unusual (and unreliable) results.
Lastly, suspended particles are often the most conspicuous of the three, comprising large particles that can sometimes be seen with the naked eye. In short, if your water looks cloudy, it’s probably not a good idea to use it in your highly sensitive assay.
2. Bacteria: tiny organisms, big problems
“In wine there is wisdom, in beer there is freedom, in water there is bacteria.” Benjamin Franklin said that, and he was right (well, it’s a significant risk at least). Bacterial contamination is a multifaceted issue, which is why it has a section all to itself. The bacteria themselves are going to be an obvious concern for anyone working with specific cultures of microorganisms (ever accidentally cultured the strain present in the water rather than the one the one you actually wanted to grow?). They can also have a negative impact on almost any other culture experiment, so always beware of bacteria!
If that wasn’t enough, bacteria can also release endotoxins and nucleases. Endotoxins are released from the membrane of gram-negative bacteria and can spell disaster for those carrying out highly sensitive processes like in vitro fertilization (both research and in practice). Nucleases can be just as damaging: as they are enzymes capable of breaking down nucleic acids, they can disrupt a whole host of molecular biology applications (that’s right, PCR is in the firing line again)!
But wait, there’s more! Bacteria populations that get a foothold in a lab water system can also form biofilms: essentially a stable colony of bacteria that surrounds itself with a self-sustaining, protective outer layer, intent on disrupting experiments by randomly releasing nucleases, endotoxins and even the bacteria themselves into the water. This problem can last for years and can be hard to fix if not tackled quickly and correctly.
3. Choosing the right purity for your application
While it’s important to understand which contaminants can influence your work, some applications are more sensitive than others. Sometimes, you just don’t need high grade, ultrapure water. For example, your bench top really doesn’t need to be wiped down with it in most cases, and I can promise you that your lab manager will not thank you for wasting it!
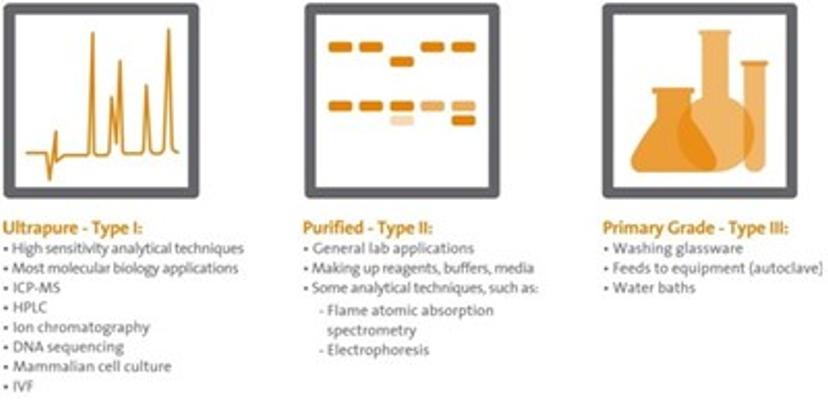
Instead, it’s important to match the right type of water with the type of application you’re working on. Washing out test tubes? Primary grade Type III water is fine. Running HPLC? You’ll need Type I ultrapure water. Preparing media? Type II purity, which sits somewhere in between these levels, will often do just fine. Using the right quality of water will not only result in robust data-generation, but it could save your lab a significant amount of money.
4. In-line monitoring for peace-of-mind
You now know about the contaminants to look out for and have a good idea about which type of water to use for your application, but how can you really be sure that your water is of a high enough quality to generate data that you can actually publish?
To provide this peace-of-mind, we’d recommend using an in-line monitoring system. By implementing a continuous sampling and testing process, you can be sure that every aliquot you dispense is of a high level of purity, without unexpected surprises hidden within!
5. Your water, on tap (so to speak)
Now you hopefully care about laboratory water as much as we do, we’re sure you’ll want to learn how you can get your hands on a steady supply, whenever you need it! More often than not, our recommendation is to install a system right there in the lab. Fortunately, these days you don’t need to worry about finding a large amount of free space for such a system (as we know, space in the lab is always at a premium!); ultra-modern water purification systems such as the PURELAB Chorus range are modular, making it easier than ever to adapt your system to meet your needs.
However, we realize that the needs of each lab can vary significantly. For example, if your lab includes a number of team members working across a number of applications then you’ll probably need a flexible set-up that can dispense all water types. To help, ELGA has created a special configurator tool so you can build a system to meet your unique requirements.
Lab water: the solution behind great science
Water may seem like an innocuous player in the world of science, but ensuring your water is of the right quality for the task at hand is essential for generating data (and findings) you can trust. Lab water specialists such as the team at ELGA are here to help you in your search for scientific insight: from providing water from everything from Antarctic surveys and research using particle accelerators, to facilitating every-day lab processes, we’re here to help support the future of science, one microliter at a time.
Jim Keary, Global Laboratory & Process Manager at ELGA LabWater, ELGA



