Lateral flow technology scale up strengthens pandemic preparedness
Discover how Merck is working to shape the future of rapid diagnostics by advancing lateral flow tests and ensuring preparedness for future pandemics
18 Mar 2025

(top left) Dr. Katie Spitere, Product Portfolio Manager, Membrane Applications and Rapid Technologies, (top right) Luis Manuel Granados, Scientific Sales Specialist Immunoassay and Molecular Raw Materials, (bottom left) Sarah Nadin, Head of Assay Development Services, and (bottom right) Roy Wu, Managing Director of Merck Life Science in China
The COVID-19 pandemic has significantly transformed the landscape of diagnostic testing, emphasizing the critical need for rapid, accurate, and accessible point-of-care solutions. Among the technologies that have seen a surge in demand and innovation is lateral flow technology. Now, as the world braces for potential future pandemics, the role of lateral flow assays is expected to expand.
Speaking with global Merck leadership in charge of its lateral flow services, we explore this dramatic shift in demand, and the steps Merck has taken to meet these evolving needs through its comprehensive and scalable product offerings, supply expansion, and support for small to midsize laboratories.
A post COVID-19 shift in lateral flow technology
The COVID-19 pandemic brought unprecedented attention to lateral flow technology, primarily due to the need for a rapid diagnostic test to help monitor and control the spread in populations. One significant trend is the decentralization of infectious disease diagnostics and the shift towards home-based testing. “The pandemic dramatically increased the demand for rapid, point-of-care testing and has spurred a massive surge in their development and production,” Katie Spitere, Product Portfolio Manager at Merck.
Designed to boost the functionality of lateral flow tests, Estapor® Color Intense Microspheres allow for the simultaneous detection of multiple analytes on a single test strip
The convenience and speed of lateral flow assays have made them a preferred choice for at-home diagnostics. Moreover, the integration of digital technologies, such as lateral flow readers and smartphone apps, is now also enabling semi-quantitative and quantitative analysis, data transfer, and storage, ensuring data integrity and enhancing the usability of these tests.
“In the coming years, industry experts predict an increase in digitalization and the use of lateral flow readers to enable quantitative analysis of the test result,” Spitere adds.
Another emerging trend agreed upon by all four experts is the introduction of multiplexing as part of lateral flow test design, which allows for the detection of multiple analytes in a single test. This approach not only reduces the overall cost per test but also provides comprehensive diagnostic information from a single patient sample. Additionally, the use of saliva as a test sample matrix is gaining traction due to its ease of collection and non-invasive nature.
“We are seeing more and more of our customers looking to develop tests with saliva. Despite being a complex matrix, lots of work has been done during and after the pandemic to optimize its use as a reliable and efficient sample type,” adds Luis Manuel Granados, Scientific Sales Specialist at Merck.
In the coming years, industry experts predict an increase in digitalization and the use of lateral flow readers to enable quantitative analysis of the test result.
Katie Spitere Product Portfolio Manager at Merck
Expanding supply to meet growing demand
With an increase in demand on lateral flow tests comes an equal demand on their components, with nitrocellulose membranes and sample and conjugate release pads being some of the trickiest to develop in house – especially for small to midsized organizations breaking into the technology. To address the increasing demand for lateral flow membranes, Merck has significantly invested in expanding its production capacity. As Spitere highlights, “Merck brought a second membrane casting line online in Cork, Ireland, in 2021 and is constructing a third facility in Sheboygan, Wisconsin, set to open in 2025. This expansion is crucial for ensuring on-time delivery, preventing supply disruptions, and maintaining competitive pricing.”

The third membrane casting facility set to open in Sheboygan in 2025
Sarah Nadin, Head of Assay Development Services at Merck, also emphasizes the importance of this expansion, noting that it not only boosts production capacity but also ensures the availability of high-quality membranes essential for the reliable performance of diagnostic tests. “We’ve expanded our raw materials capabilities, and I think this is a really positive thing because it allows us to respond to these trends more quickly,” Nadin states.
Roy Wu, Managing Director of Merck Life Science in China, also highlights the strategic importance of these expansions. “We have clearly observed a rise in Chinese companies developing lateral flow devices post-COVID-19, and the increased capacity will ensure we are able meet the growing demand in this market,” he explains. “This move positions Merck as a leader in providing the necessary infrastructure to support rapid diagnostic manufacturers globally.”
Enabling success in small to midsize labs
Merck’s commitment to supporting small to midsize laboratories is evident in its comprehensive service offerings. The company provides contract research and development services, raw material supply, assay development services, as well as training and education programs. These services are designed to assist smaller firms in developing and bringing lateral flow tests to market efficiently and cost-effectively.
Granados emphasizes that Merck’s infrastructure and expertise enable small companies to navigate the complexities of developing lateral flow assays, from proof of concept to commercialization. “We often work with scientists who have a great idea for a lateral flow test, and by partnering with Merck, they can leverage our expertise and resources to bring their product to market much faster,” he shares.
Nadin echoes this sentiment, highlighting the role of Merck’s assay development teams in supporting smaller companies. “We have grown dramatically during the last few years, expanding our team of diagnostics experts to North America and Asia, which allows us to collaborate closely with customers at any scale globally and help them overcome challenges quickly and efficiently,” she explains.
Serving lateral flow test utility beyond infectious disease
Beyond human diagnostics, Merck is leveraging its expertise to expand into other customer segments, such as veterinary diagnostics, food safety, and environmental testing. This strategic expansion is driven by the growing need for rapid and reliable testing solutions in these areas. For instance, the detection of pathogens in food and water supplies or monitoring disease outbreaks in livestock requires quick and accurate diagnostic methods, making lateral flow assays an ideal choice.
“COVID-19 made the public aware of lateral flow technology, and now more companies are developing tests for other infectious diseases and even environmental monitoring,” Wu notes. “The pandemic has demonstrated the importance of having robust and flexible diagnostic capabilities that can be deployed quickly and effectively in diverse settings.”
Granados also points out the increasing interest in quantitative and multiplexed tests, particularly in markets like veterinary diagnostics and food safety. “More and more customers are interested in moving towards multiplexing and quantitative tests, which offer greater sensitivity and specificity,” he states. “This shift is indicative of the broader application potential of lateral flow assays beyond traditional uses.”
Providing a reliable platform for future innovation
The COVID-19 pandemic has undeniably reshaped the landscape of diagnostic testing, underscoring the importance of rapid and accessible point-of-care solutions. Lateral flow technology, with its versatility and ease of use, is poised to play a pivotal role in future pandemic preparedness and beyond. With diagnostic specialists like Merck placing a strategic focus on offering complete lateral flow solutions, expanding supply capabilities, and supporting small to midsize laboratories, the right steps are being taken to ensure reliable platforms are available for continued innovation in this sector well into the future.
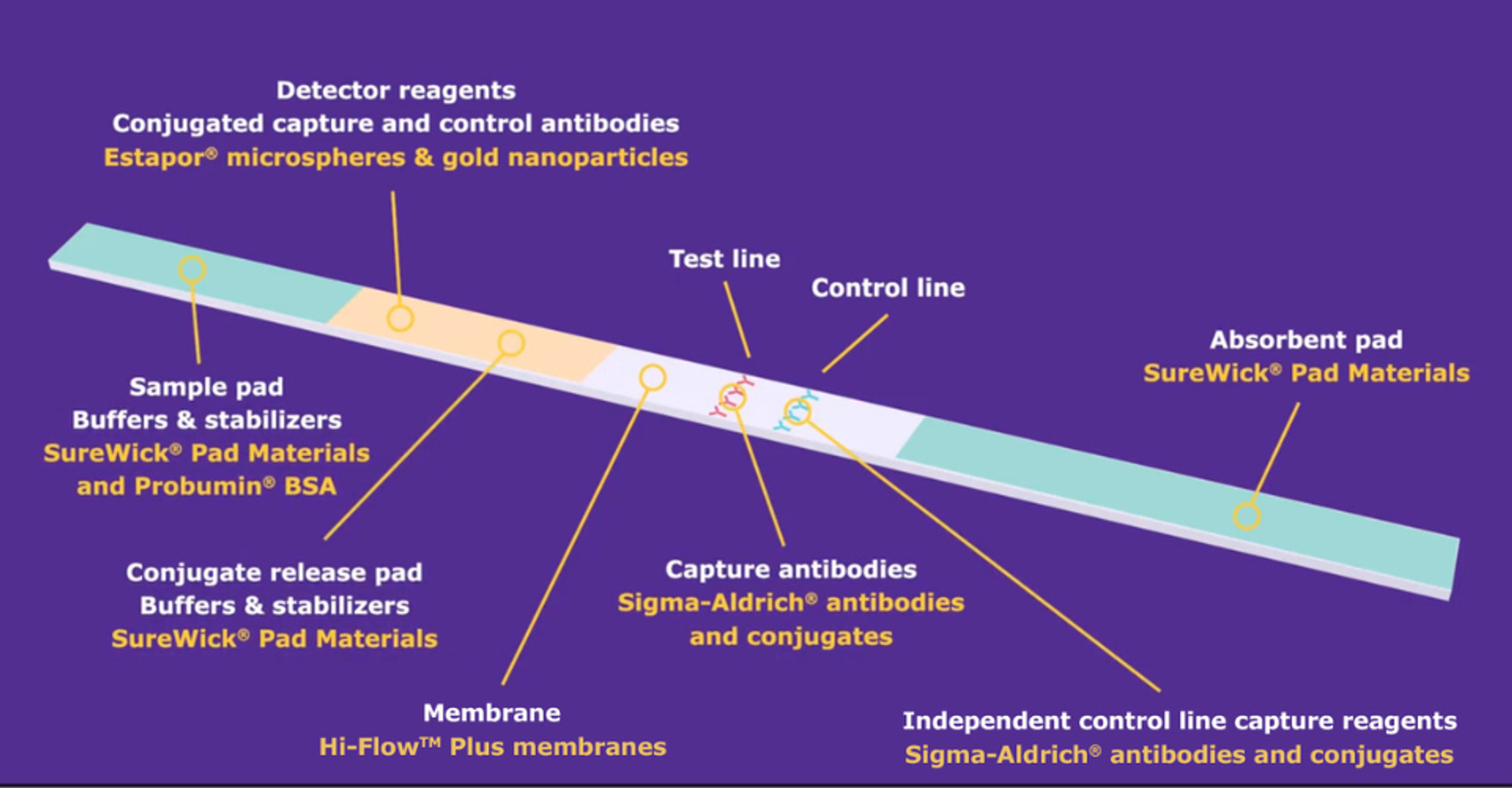
Merck offers all other raw material components required for lateral flow test manufacture including pads, detector particles, buffers, blockers, and antibodies.
As Nadin summarizes, “Our ability to make more of our high-quality membrane is what’s special about this. It’s not just about more membrane in the market, it’s about ensuring that our customers can rely on consistent, high-performance materials to develop their tests.”
“Merck offers all other raw material components required for lateral flow test manufacture including pads, detector particles, buffers, blockers, and antibodies,” Spitere adds. “This comprehensive approach simplifies the supply chain for diagnostic manufacturers and ensures that all components work seamlessly together.”
Request a free sample pack to see how Hi-Flow™ Plus membranes can enhance your tests.