Leveraging multi-omic technology to better understand cognitive dysfunction
Learn how a group of MIT researchers are using novel assay technology to better understand anterior thalamic dysfunction
1 Aug 2022

Neuropsychiatric disorders are often accompanied by cognitive impairments or intellectual disability. However, it is not clear whether there are converging mechanisms underlying these debilitating impairments.
In this SelectScience® article, Dr. Dheeraj Roy, Broad Institute of MIT and Harvard, discusses his research identifying converging cellular to circuit mechanisms underlying cognitive deficits in the anterior thalamus. Roy also explores how novel multiplex fluorescent assays have enabled his team to overcome challenges in understanding cell populations in this area of the brain.
Dr. Dheeraj Roy recently presented his work in the SelectScience® webinar series: Diversity, development, and dysfunction in the brain. Watch his presentation on demand, here >>
What are the overall goals for your research?
DR: I wanted to study a region of the brain, which is understudied, and the thalamus is one such region. From human work, we already know that patients with lesions that are restricted to the thalamus have all sorts of cognitive deficits, but no one talks about the thalamus as one of these important cognitive centers.
We use mice for all my research, and we've picked a few thalamic nuclei, such as the anterior thalamus to study. It happens to have three subdivisions, and these are not simply some sort of sensory relay to the rest of the cortex but are actively processing and contributing to learning activities whether that be encoding new information or distinguishing between two experiences. My goal is to examine understudied brain regions and reveal their functional contributions. I'm very excited to try and bring this knowledge to disease models, to see if we can learn something about if these brain regions are altered by disease.
What are the key challenges you face when investigating the anterior thalamus?
DR: The first thing is, unlike other structures in the brain, we don't know very much. We know that it exists in the atlas, and we know some information about its’ crude anatomy. What we don’t know is if there are distinct cell types present and if there are related cell types that work in a complementary fashion, mainly because not many researchers have done work to characterize them. We also don’t know if they are distinct across age or if they are altered in disease states.
Without a catalog of the structure (i.e., what sorts of cells and neurons are present), it's very hard to explain what the region is doing and how it's doing it. Our first challenge was making a version of this catalog to just explain what we were looking at.
Once we have a catalog of the structure, we can start developing either a mouse line or a viral approach to start studying the region with some selectivity to assign a function to the cell populations, and this is the biggest challenge. I think for any brain region, you have a similar issue with regards to finding the right tools to interrogate the structure (understanding what the components are), and then try to functionally test what their role is in behaviors.
How do you use RNAscope technology in this work?
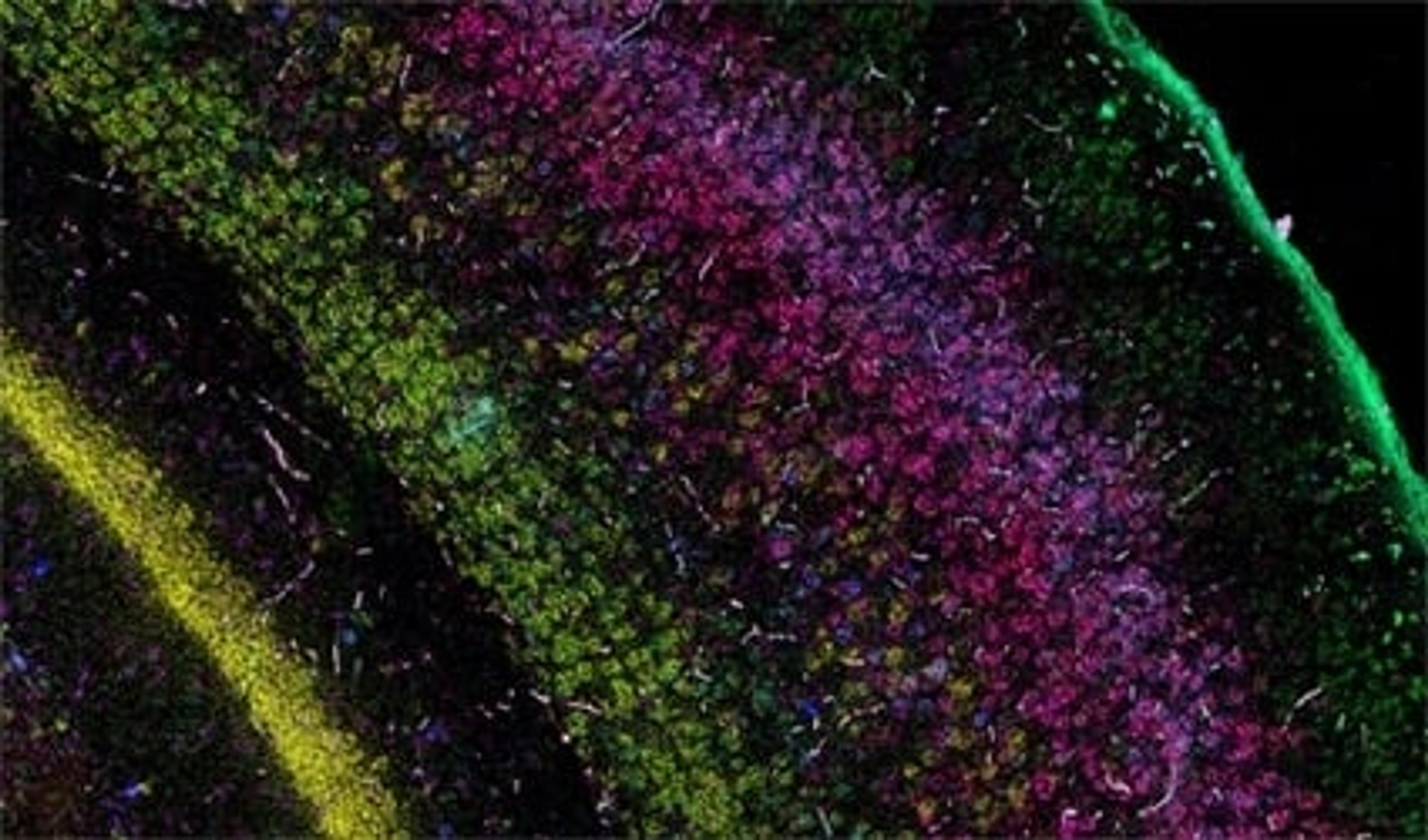
DR: We've generated RNAseq data and we now have these massive gene lists for potential cell types. In the old days, you would go one gene at a time, and with previous fewer sensitive techniques, you would miss a lot of marker genes. We use the RNAscope™ Multiplex Fluorescent Assays by Advanced Cell Diagnostics where we can probe for extremely weak or strong targets in the same tissue with the same kit and with one type of probe design. With the assay, you can probe four genes at once, and I also know examples of where you can do twelve, but at least for our work being able to study four genes simultaneously has been amazing. From our RNA sequencing data set for our brain region, I can now go and start screening which markers are restricted to the different subdivisions of the anterior thalamus.
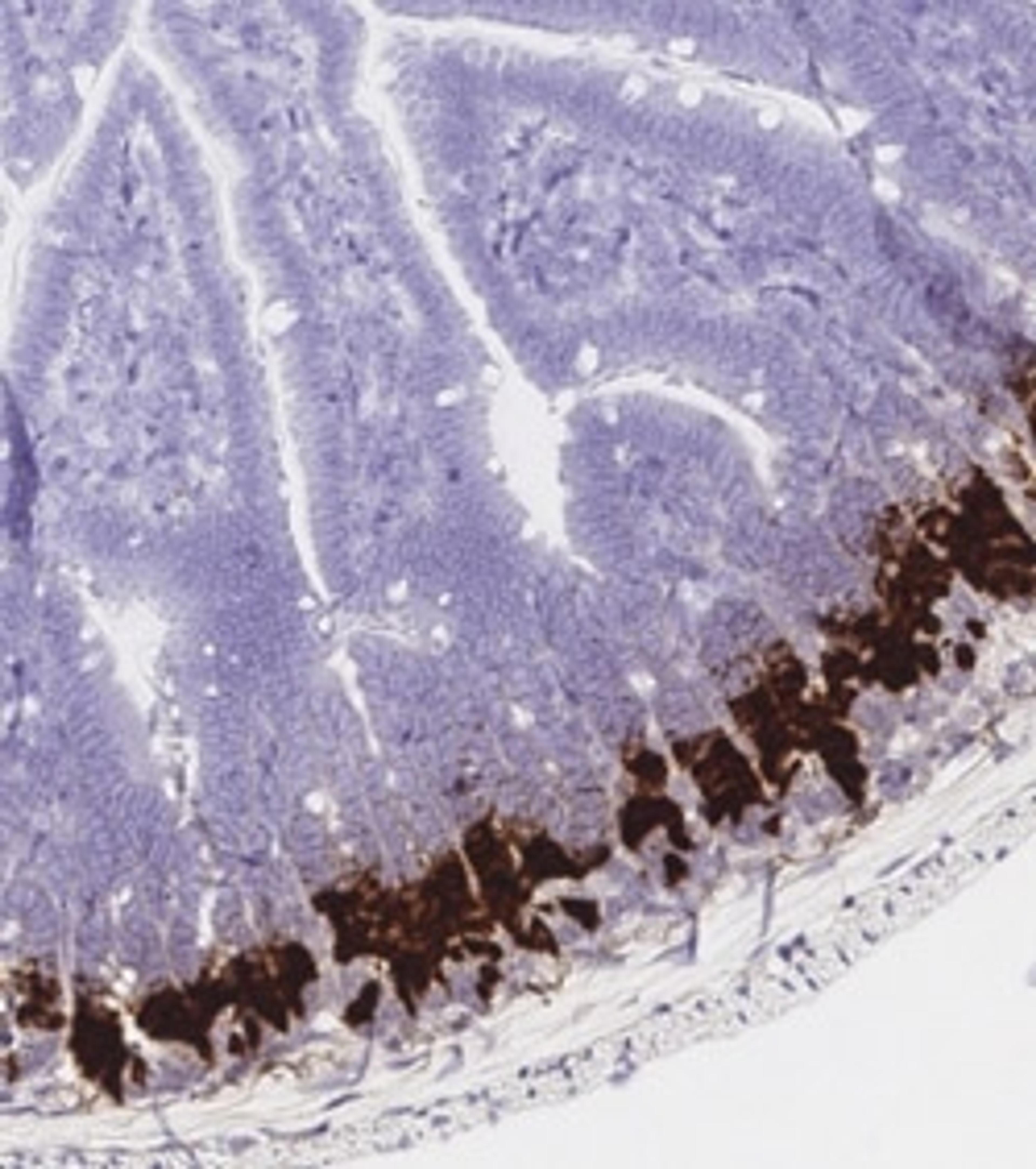
We're also using the RNAscope™ Chromogenic Assays by Advanced Cell Diagnostics to directly look at the human thalamus. Now we're looking for the cells and the markers that are shared between mice and humans, and I can use this information in a mouse to test functionality.
What does this technology enable you to achieve that you couldn't before?
DR: Previously you'd get maybe eight out of twelve of your gene candidates. However, some would not be visible because the gene levels are lower. With RNAscope, what we noticed is even for these weaker genes which we couldn't see before, now we can. Previously, every time you would do this using three or four-day protocols, you'd typically only get two pieces of information for two different genes. Now we're able to study four genes at once in a one-day protocol.
What are you most excited about for the future of your work?
DR: We now have a list of genes that we think are good candidate markers for distinct cell types in the anterior thalamus. What we've been doing is now making cell lines to study it and depositing these online for people to share. What this allows us to do is to start assigning functions to each cell type. For example, a C1QL2 cell type in the anterior thalamus we think is important for contextual encoding. However, C1QL2 negative neurons also express a different marker called COL25A1. Again, with the help of RNAscope, we've assigned it the function of distinguishing between two similar memories. We need to continue to understand the different things these cells are doing separately as well as together. And then, look at what disease states are doing to these cell types.
Why is peer-to-peer communication in science important?
DR: Whenever there is a group of people that has a particular project or question, the goal is not to just do something in isolation. It is important to get your work out there at the earliest stages. For me, webinars, articles, and participating in conferences are the best ways to get your research out into the wider scientific community. It's exciting to be able to tell people what's not available in the textbook.
I think the nice thing that comes with sharing your work is you get feedback. You'll always have a handful of people that can tell you things that you just did not think about. Maybe it may look like criticism, but it's really good feedback and it'll take your research to the next level. And then finally, I think science communication is important to build your reputation in your field and is also very helpful when looking to find collaborators.