Liquid Biopsies Guide Individualized Treatments for Lung Cancer Patients
Find out how Biodesix is using Droplet Digital™ PCR to help physicians and patients make informed treatment decisions
28 Aug 2017

Biodesix discovers and commercializes breakthrough cancer tests that use a patient’s unique molecular profile to inform treatment decisions. These tests help oncologists to make precision medicine decisions that improve patient care. SelectScience® speaks to Dr. Hestia Mellert, Director of Molecular Product Development, Biodesix, to learn more.
SS: Could you start by telling us about Biodesix and your job role there?
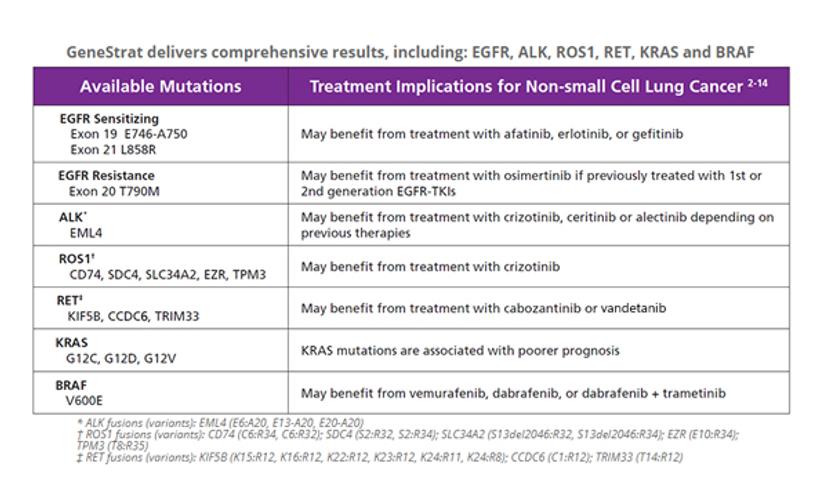
HM: My main focus as the Director of the Molecular Product Development group is to lead cross-functional teams in the development of our blood-based nucleic acid diagnostic tests. The GeneStrat RET and ROS1 tests were our most recent products and these were launched earlier this year. Collectively, the GeneStrat® tests measure the most prevalent somatic variant mutations and gene fusion transcripts that arise from tumors of lung cancer patients. Specifically, the GeneStrat tests are focused on the detection of actionable mutations from circulating DNA and RNA present in plasma.
A liquid biopsy test
SS: Can you tell us more about the GeneStrat test?
HM: The GeneStrat test is a liquid biopsy test that can provide physicians and their patients with actionable molecular information quickly. The test relies on a minimally invasive blood draw and is shipped at ambient temperature to our CAP/CLIA certified laboratory in Boulder, CO. The GeneStrat test has also been recently approved by NYS CLEP and includes the detection of variants in EGFR, KRAS, BRAF, EML4-ALK, RET and ROS1. Circulating nucleic acids are extracted from the plasma and analyzed using the Bio-Rad QX200 ddPCR™ System. The process has been optimized to deliver test results to the ordering practice within 72 hours of sample receipt.

SS: What are the advantages of using ddPCR for this test?
HM: A tissue biopsy is an invasive procedure that carries risks including bleeding into the lung, infection and pneumothorax. In addition, approximately 25% of the patients are either poor candidates for tissue biopsy or may not have sufficient tissue for molecular diagnostic tests to be run accurately. Due to logistical constraints, results from tissue biopsies may take several weeks to be returned to the treating physician. Liquid biopsy allows for the sensitive detection of rare tumor-derived nucleic acids using a minimally invasive blood draw.
The Bio-Rad QX200 ddPCR™ system is ideally suited for liquid biopsy because the technology allows for detection of rare tumor-derived mutations in a dense background of wild-type sequences. It is also much faster than many alternative liquid biopsy tests that currently use a next-generation sequencing (NGS) approach. This is a key differentiator for GeneStrat, and a feature that our physicians really love about the test: it is rapid and accurate.
Informing individual treatment decisions
SS: What benefit does GeneStrat offer to the patient?
HM: The physicians that use the GeneStrat test may be able to provide their patients with an actionable result within days of ordering and drawing the blood. This allows physicians and patients to make more informed and individualized treatment decisions, and can lead to patients receiving optimal treatment much faster than the current standard of care diagnostic options.
SS: What are your future plans for the technology and/or tests offered by Biodesix?
HM: We are very excited to see the promising results of immunotherapies in lung and other cancers. However, we are also acutely aware of the continued complexity of treatment decisions, specifically in terms of determining which patients will likely benefit from immunotherapies either alone or in combinations. Biodesix is committed to the development of diagnostics that can help to further refine decision-making using both our molecular and proteomics-based approaches. This is particularly important for immunotherapy, since treatments can cause severe adverse events and are a heavy financial burden for families and the health care system.
Visit the SelectScience product directory to learn more about the QX200 ddPCRTM System