Malaria diagnostics and testing: Opportunities and challenges
Discover the latest in malaria diagnostics, from currently available techniques to the implementation of effective disease management strategies
16 Jun 2022

Dr. Jane Achan is a medical doctor and senior research advisor at the Malaria Consortium with an active involvement in the epidemiological surveillance of malaria morbidity and mortality trends. Dr. Achan also provides technical advice on case management policy issues. This article summarizes the key learnings from Dr. Jane Achan’s fascinating talk at the SelectScience® Virtual Microbiology and Infectious Disease Summit 2022, now available to view on demand. Achan’s talk provides an overview of the different diagnostic approaches that are available and explores the opportunities and challenges of current malaria diagnostics and testing techniques.
The need for better malaria diagnostics
Malaria is a life-threatening disease that is transmitted to humans through the bites of infected female Anopheles mosquitos. The parasite causing malaria is named Plasmodium, 5 types of which can infect humans. About 3.3 billion people, or half of the world’s population, are at risk of malaria, with the majority of cases in sub-Saharan Africa.
Currently available point-of-care malaria diagnostic tests for routine use
Malaria infection ranges from symptomatic infection to asymptomatic parasitemia, and where individuals are placed on this spectrum can infer distinct diagnostic strategies. Symptomatic infection presents in semi-immune to non-immune people such as children, tourists, and residents in low-transmission settings. This class of infection generally has a high parasite load and can be detected and diagnosed by microscopy or rapid diagnostic tests (RDT). The diagnostic goal for symptomatic infection is individual case management, identifying appropriate treatment strategies for each patient. Asymptomatic parasitemia can be described as a ‘carrier’ state and is much more prevalent in endemic zones than was previously recognized. This is a subject for concern as carriers of malaria form a reservoir of transmission. Carriers of malaria are detectable by molecular and serological methods, and the aim for detection is to screen for elimination.
As the presentation of malaria infection can differ, it is important that diagnostic methods meet the unique requirements between cases. The sensitivity, specificity, and limit of detection of each diagnostic technique all have implications in how, and in what setting that technique should be implemented.
Microscopy: Lighting the way for malaria diagnostics
The gold standard of malaria diagnostics is currently the visual detection of parasites in stained blood smears through light microscopy. This method enables the quantification of parasite present in the sample and allows scientists to distinguish between species and life cycle stages. This method is simple, accurate, and inexpensive, with the sensitivity ranging from 30-50 parasites per µL to 50-500 parasites per µL. However, this method is labor intensive, and the accuracy depends heavily on the skill level of the laboratory staff.
Fluorescent microscopy is a modification of light microscopy that can also be used for malaria diagnosis. As mature red blood cells do not contain genetic material, this method relies on the detection of parasitic genetic material using fluorescent dyes. Certain techniques that utilize fluorescence microscopy can increase sensitivity by around 90%, even in routine settings.
Malaria antigen detection: Rapid diagnostic tests
Rapid diagnostic tests (RDTs) are the most commonly used diagnostic technique in primary care settings and play a key role in diagnosing suspected malaria cases across the globe. Antibodies are used to detect one or several parasite-specific antigens, such as Plasmodium histidine-rich protein 2 (HRP-2) and Plasmodium lactate dehydrogenase (pLDH), within a blood sample, through lateral flow immunochromatography.
RDTs have become an invaluable tool in the detection of malaria for several reasons. The test is highly sensitive and specific, the technique is easy to perform, and the results are fast and easy to interpret. This means the tests are suitable for community-level health facilitates in rural areas such as in sub-Saharan Africa. However, RTDs may fail to detect malaria in cases with low parasitaemia, and false positives are a concern due to cross reactions or delayed clearance of HRP-2 after treatment.
Highly sensitive RDT (HS-RDTs) were developed for the field detection of low-density infections. These tests have a detection limit that is tenfold lower than that of conventional malaria RDTs. However, studies suggest that HS-RDT sensitivity is generally low, missing 62% of infected individuals identified by PCR (Mwesigwa et al., 2019). This highlights the need for point-of-care (POC) molecular diagnostics especially in low transmission settings, which appear to have unique diagnostic needs compared with high transmission settings.

The hidden reservoir: Asymptomatic, low-density infections
Nucleic acid amplification-based tests (NAAT)
Individuals with asymptomatic parasitemia or low-density infections present a unique challenge to malaria diagnostics. This group calls for NAATs as these tools have been very effective in estimating current malaria infection, provide better detection of low limits of infection, can discriminate species, and quantify parasitemia. This suggests that NAATs have the potential to overcome several hurdles for malaria diagnostics at the POC.
Polymerase chain reaction (PCR)
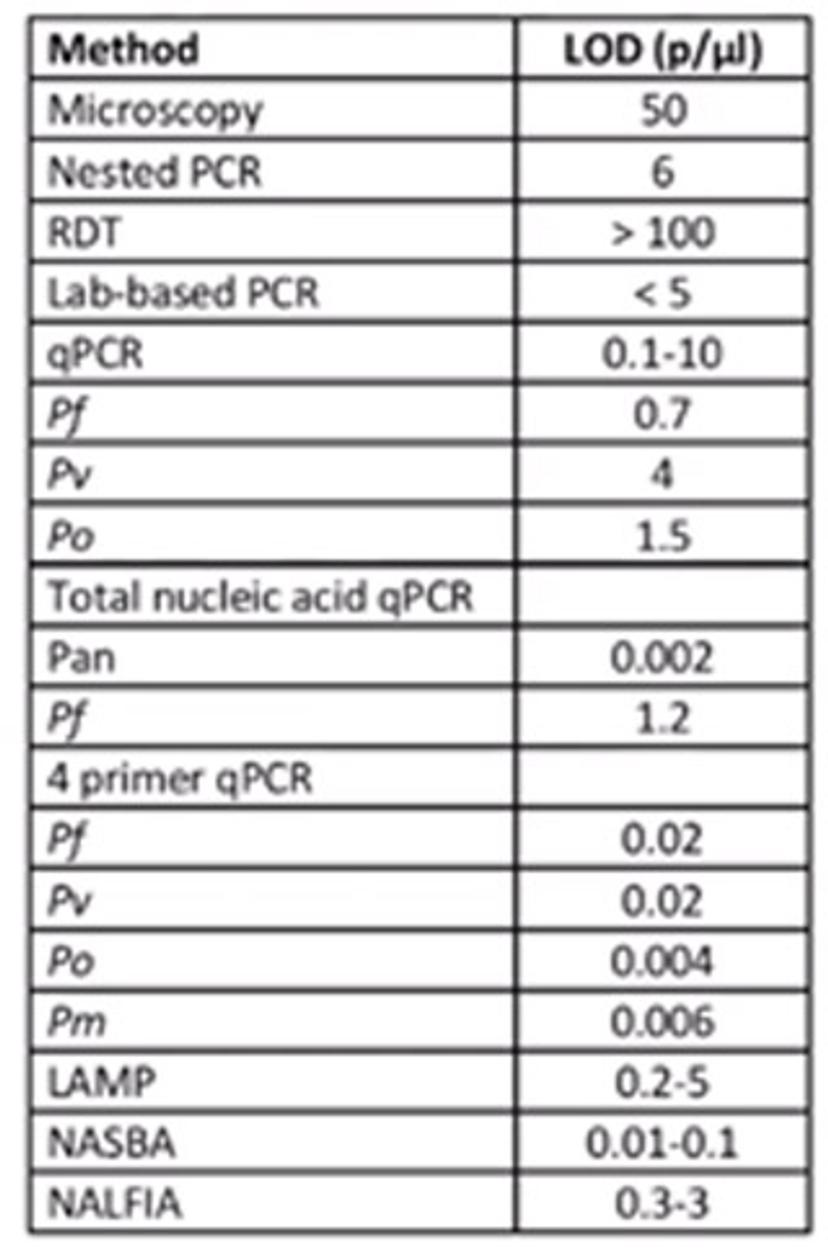
One of these approaches is PCR, which provides a sensitive diagnostic tool for both detecting and quantifying parasite DNA. There are several PCR techniques available. Nested, or conventional, PCR involves the amplification of specific parasite targets, whereas quantitative real-time PCR (qPCR) requires the use of labelled probes for increased specificity. Multiplexed PCR is a technique in which multiple target sequences are amplified simultaneously in a single reaction well, with a different pair of primers for each target. For all of these approaches, the limit of detection depends largely on sample preparation and extraction efficiency, the amount of blood available, and the sample and storage format that is being used.
Lacewing technology: Portable technology for rapid detection of infectious diseases and outbreaks in real-time
These NAAT techniques are of interest for the development of new POC or handheld molecular diagnostics. The Lacewing technology is one of these developments that has caused excitement in the malaria research community. It is a portable technology for rapid detection of infectious diseases and outbreak in real-time. This is currently being developed by Imperial College London in collaboration with different partners. The technique largely involves having a disposable cartridge onto which the extracted patient sample is placed and a handheld diagnostic device, within which the real time DNA amplification occurs. The outputs of this amplification are then displayed on a smartphone application. This has been tested for several infections, but particularly for malaria it has been shown to produce promising results.
Isothermal amplification methods
Isothermal amplification is another method for malaria diagnostics. Loop-mediated isothermal amplification of DNA (LAMP) is one such technique. This is a simple, sensitive, and rapid DNA amplification system that performs under isothermal conditions, meaning that no thermocycler or gel imaging detection system is required. Specificity is high for all malaria species, and there are on-going efforts to optimize the specificity, sensitivity, and high-throughput format of this method. The use of LAMP is key for malaria testing and is utilized in settings beyond the lab, such as in pre-elimination settings, national surveillance and epidemic detection efforts, field antimalarial efficacy monitoring and vaccine trials, and detecting placental malaria.
Novel non-invasive malaria diagnostics
There has also been a key interest in non-invasive samples and detection methods. One sample type that has been utilized is saliva. For saliva-based detection there are two approaches: nucleic acid amplification and Plasmodium protein detection. The saliva-based test with nucleic acid amplification detects the Plasmodium gene, 18S rRNA or the P. falciparum dihydrofolate reductase gene in saliva using a nested-PCR. The sensitivity and specificity of this method ranges from 86.36% to 95%, and the limit of detection is 1-10 parasites per µL of blood. This method holds advantages beyond the fact it is non-invasive, in that it requires little training for health personnel to collect samples. However, it does involve the use of PCR which requires advanced training. Furthermore, the procedure takes approximately 6 hours, which is a major hindrance for its implementation.
The saliva-based Plasmodium protein detection method detects the presence of specific proteins in saliva. There are currently kits commercially available, such as the OptiMAL-IT® dipstick (RDT) which detects pLDH in the saliva. Field trials have been carried out with varying sensitivity, ranging from 78% in Nigeria, to about 98% in Mali when compared to microscopy. One particular challenge is that this method has a very high limit of detection at around 1000 parasites per µL of blood. This is a major limitation as it may potentially miss a large number of infections that have lower density parasitaemia. Therefore, further tests would be required to confirm any negative results. The key advantage of this is that it is non-invasive.
Urine
Another non-invasive sample is urine, and urine-based malaria tests have also been developed. This involves the detection of Plasmodium protein pHRP-2, and the commercially available test, the urine malaria test (UMT), involves dipping a test strip into a urine sample, followed by a 20-minute incubation. A positive result is indicated by dark-colored lines on the test strip. The UMT has been tested in a multicentre clinical trial in Nigeria among febrile and afebrile patients. The sensitivity and specificity when compared to a routinely used malaria diagnostic test, BinaxNOW® Malaria Test Kit, was 79% and 89% respectively. The limit of detection for this method is 125 parasites/µL, and each test costs ~ $1.50. The affordability of this test is a main advantage and does not require expensive equipment or highly trained personal. A key limitation, however, is that it only detects pHRP-2 from P. falciparum parasites.
Transdermal hemozoin detection
How does it work?
An excitatory pulse is administered to blood vessels through the skin
This localizes heat and evaporates liquid around the hemozoin crystals
The liquid evaporation creates expanding and collapsing small sized vapor nanobubbles inside the malaria parasite
These movements are detected by a probe and displayed as an electrical signal or acoustic trace
The trace will present differently for malaria positive and malaria negative individuals
Transdermal hemozoin detection involves the detection of hemozoin-generated vapor nanobubbles using an ultrasound sensor. Hemozoin is a by-product of hemoglobin digestion by blood-stage malaria parasites. Hemozoin clears from the blood within 9 days, compared to pHRP-2 which is cleared after several weeks, reducing the probability of false positive results. This technique requires no reagents and results can be obtained within seconds. Furthermore, it has a very low limit of detection and may potentially detect subclinical carriers which is valuable for disease surveillance. However, this method is still under development, requires highly trained personnel in both clinical and field settings, and the equipment costs are high. Skin color has also been found to impact on results, and more studies are needed to address this issue.
Challenges and considerations in malaria diagnostics
Malaria is predominantly a disease of children under 5, and this is where a lot of the death and illness occurs. Timely malaria diagnosis and treatment is critical to prevent this mortality, and good quality diagnostics are vital for this.
Overall, there are several key challenges that continue to hinder malaria diagnostics. Among these is the diagnosis of asymptomatic carriers as this group requires highly sensitive malaria diagnostics. Asymptomatic carriers are 4-5 times more prevalent than symptomatic infections in endemic settings, hinting at the significance of targeting this group to tackle the burden of disease.
Another key challenge is malaria diagnosis in elimination settings. In this case, there are really low levels of parasitaemia that require highly sensitive malaria diagnostics. These settings also need to have fully functional surveillance and response systems that can detect and prevent re-establishment of indigenous transmission.
The diagnosis of malaria during pregnancy remains a challenge. Diagnosing placental malaria is especially challenging as parasites sequester in the placenta and cannot be accurately detected by microscopy in the peripheral blood. RDTs that detect pHRP-2 are useful tools in this context.
As the scientific community reviews the current methods of malaria diagnostics, the techniques must be considered in the context of the existing challenges that continue to hinder disease management and should be customized depending on the priorities of each unique challenge.
Watch Dr. Jane Achan’s full presentation on malaria diagnostics from the Virtual Microbiology & Infectious Disease Summit on The Scientists’ Channel
References
Cordray M, Richards-Kortum R. Emerging nucleic acid-based tests for point-of-care detection of malaria. Am J Trop Med Hyg. 87(2), 223-230 (2012). doi: 10.4269/ajtmh.2012.11-0685
Mwesigwa J, Slater H, Bradley, J. et al. Field performance of the malaria highly sensitive rapid diagnostic test in a setting of varying malaria transmission. Malar J, 18, 288 (2019). https://doi.org/10.1186/s12936-019-2929-1
Vasoo S, Pritt B, Molecular Diagnostics and Parasitic Disease, Clinics in Laboratory Medicine, 33(3), 461-503 (2013).
