Medimetrics Expands Medical Device Portfolio with New Fast Release IntelliCap
17 Sept 2014
Company Wins ISO and EN Certification for its Design & Development Capabilities
Medtech innovator Medimetrics continues its expansion into the global medical devices sector with official recognition of its full design and product development capabilities and the launch of a new, fast release, drug delivery capsule.
Medimetrics has newly achieved a certification of compliance to EN ISO 13485:2012. This enhanced level of certification further confirms the company’s expertise in the design, development, manufacturing and customer support of products and services for personalized drug delivery.
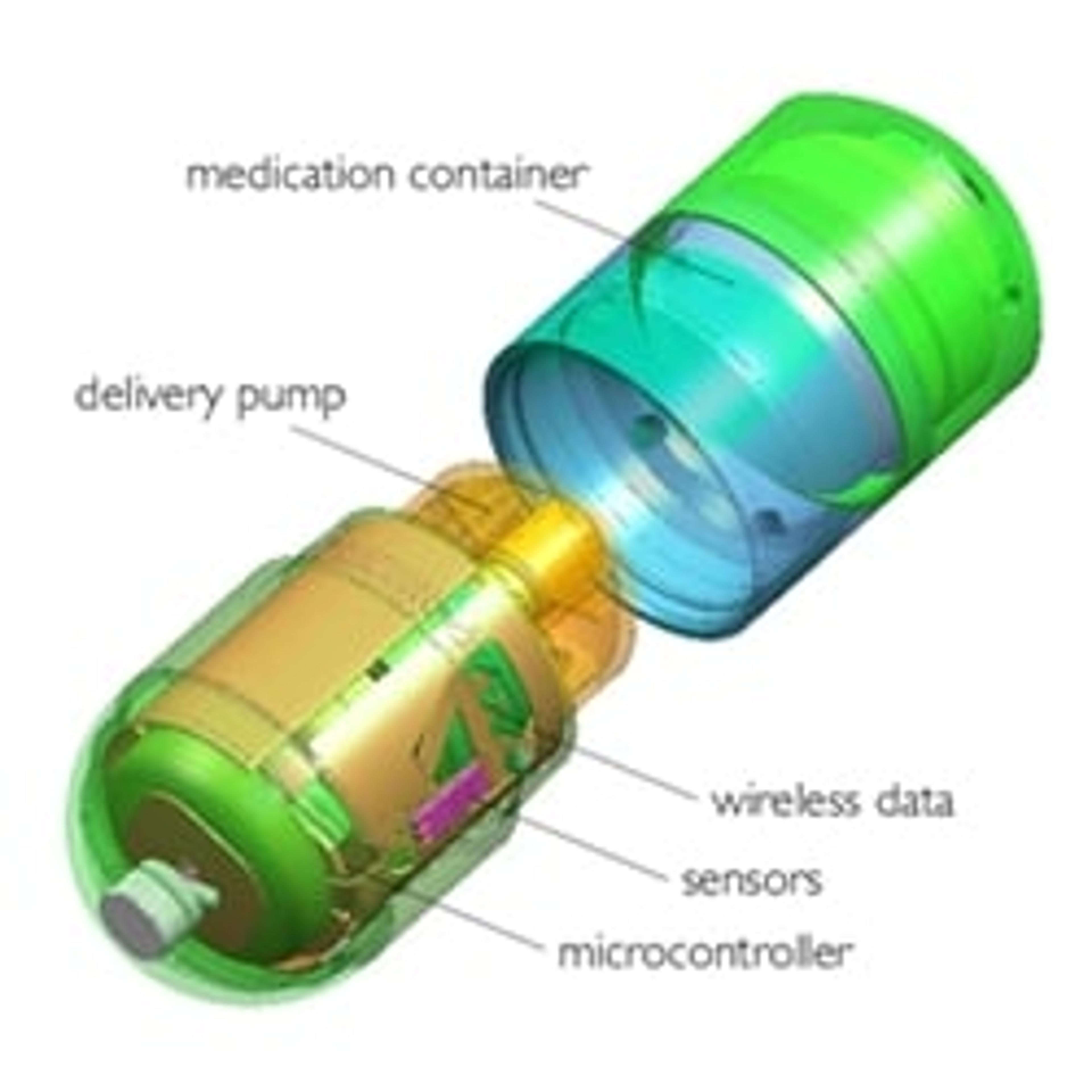
IntelliCap FR system
Official recognition of its design capabilities comes as the company launches its new product, the IntelliCap FR system, which has just been granted its CE Mark. The new IntelliCap FR system is a digitally operated capsule suitable for use in clinical trials to deliver drugs in the gastro-intestinal tract, along with measuring and reporting conditions in the body.
“Digital innovations are leading the way in helping bring about a more personalised healthcare,” said Medimetrics chief executive officer, Dr Olaf Weiner. “Medimetrics is already working with five of the world’s leading pharmaceutical companies on controlled drug delivery with the IntelliCap CR system. Its success has led customers to ask us to go a step further and create a different type of digitally operated product, but one still based on the unique aspects of IntelliCap. They wanted a device that had the ability to delivery any type of drug payload, including solids and powders.”