Microglia Prune Synapses and Shape Neural Circuits During Development
Performing more than just as immune responders, the understudied glial cells, the microglia, regulate synaptic connectivity during development and disease
23 Jul 2018
Glial cells constitute over 50% of cells in the brain, and yet, they remain heavily understudied. Neurons, the popular residents of the brain, have long basked in the limelight of scientific attention, overshadowing the importance of the glia, contributing to their label as the brain’s passive ‘supporting cells’. More recently, the glia finally got their due, rising into the focus and revealing roles far greater than mere support.
In this SelectScience® interview, we speak with Dr. Dorothy Schafer, Assistant Professor, University of Massachusetts, whose primary interest is understanding the function of glial cells, especially a subset of glia called the microglia. Schafer also discusses the importance of designing good experiments and choosing reliable antibodies for studying a mechanism never before explored.
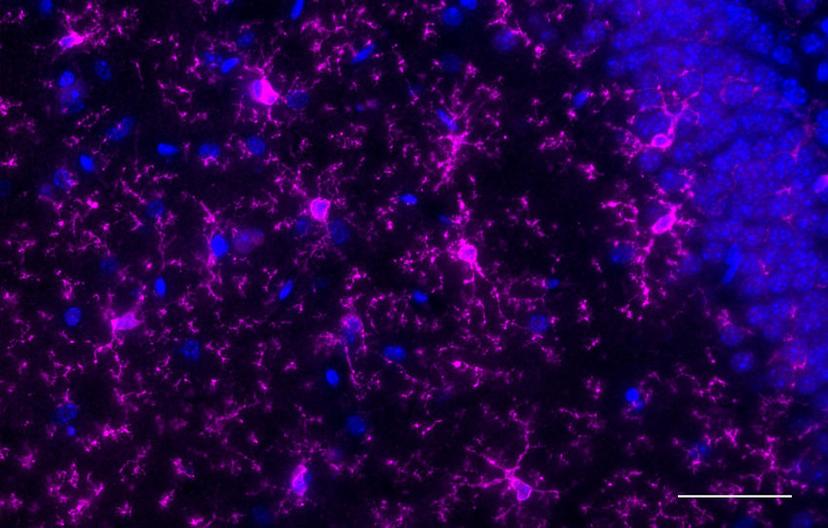
As the brain’s immune cells, it is no surprise that the microglia are involved in disease states. Recent studies have implied their role in brain health as well – an opportunity that Schafer’s lab pursues. “They really were these mysterious cell types for a long time – we didn’t appreciate fully that they could be performing important functions in the healthy brain,” admits Schafer. “And now it’s becoming increasingly clear that they, in fact, do play an important function in shaping the developing nervous system.”
The Schafer lab studies how microglia sculpt neuronal circuits – a huge functional step-up from simply providing support. “We’ve been working on how microglia regulate the plasticity of synapses and neural circuits in response to changes in sensory experience,” says Schafer. “We then apply these mechanisms to diseases of the nervous system such as Alzheimer’s disease or schizophrenia.”
They really were these mysterious cell types for a long time – we didn’t appreciate fully that they could be performing important functions in the healthy brain.
Dr. Dorothy Schafer University of Massachusetts
Schafer focused on the glia very early on. As a graduate student, she studied another type of glial cell, the myelinating glia, and explored its function in demyelinating disease. As a postdoc, she expanded to studying the role of microglia in synaptic remodeling. Schafer used the visual system as a model for testing how microglia help fine-tune or prune neuronal connections.
“My postdoc mentor, Dr. Beth Stevens, had identified that mice deficient in innate immune-system related proteins – the complement molecules C1q and C3 – had defects in synaptic pruning in the developing visual system,” Schafer explains. “These are innate immune molecules involved in the body’s defense to fight infection – how they regulate synaptic pruning was a real mystery.”
Schafer then identified that there was one player that linked the immune molecules to synaptic pruning – the microglia. “When I was in the Stevens’ lab, I identified that microglia, through receptors of complement proteins, were engulfing synaptic elements,” says Schafer. The microglia engulfed synapses, thereby pruning and eliminating unnecessary synapses in the visual circuitry, via the complement pathway. “When we blocked this pathway, we found that mice had long-term defects in pruning with sustained increases in the number of synapses in their brain.”
The results of this study were published in Neuron, 2012. Along with a few other publications at the time, this was the first evidence that microglia were really involved in sculpting circuits in the developing brain under healthy non-pathological conditions. With the honor of being the first to observe findings comes a big responsibility – choosing relevant experiments and specific antibodies.
Experiments that study cell-cell interactions and measure synaptic elements require finesse and high specificity. “We use a lot of immunohistochemistry (IHC) and fluorescence microscopy both in situ and in postmortem and tissue culture samples to look at microglia-neuron interactions. We also combine that with two-photon live imaging to look at microglia-synapse interactions in real time.”
The Schafer lab uses BioLegend antibodies for IHC and immunofluorescence experiments. “Two important factors determine our antibody choice. One, that it’s specific. And two, that we can visualize immunofluorescence within our tissue with a good signal to noise,” explains Schafer. “Price certainly comes into the factor, and that’s where BioLegend has been really nice,” Schafer continues, “by offering smaller sizes of antibody samples for us to try out.”
Two important factors determine our antibody choice. One, that it’s specific. And two, that we can visualize immunofluorescence within our tissue with a good signal to noise.
Dr. Dorothy Schafer University of Massachusetts
“Good technical support has also been key for us in deciding what antibodies to use. The BioLegend representative who I met at the immunology retreat at UMass has been really helpful in sending us samples and letting us try out antibodies before we actually buy them.”
Reliability forms the defining factor of choice for Schafer. “If we want to immunolabel microglia in fixed tissue or FACS sort microglia and look at their phenotype in response to any of our perturbations, we use BioLegend antibodies. These have been very reliable for immunofluorescence confocal microscopy and FACS,” says Schafer.
Schafer expands on her choice of antibody: “For immunofluorescence, we have used P2RY12 and PECAM antibodies. We used anti-PECAM to visualize vascular endothelial cells because we have a project on assessing microglia-vascular interaction, and anti-P2RY12 (purinergic receptor) to distinguish microglia from peripheral monocytes, which has been working really nicely. For FACS we’ve used CD11b from BioLegend conjugated to different fluorophores. Microglia are generally CD11b high- and CD45 medium-expressing. So, I’ve used these as markers for FACS to distinguish microglia from peripheral monocytes or macrophages, and ITGB5 for astrocytes.”
In her recent project, Schafer delves deeper into microglial engulfment of synapses. “In my lab, we’ve identified that microglial engulfment is dependent on the animal’s sensory experience. We can manipulate the somatosensory system to test this: if we removed whiskers from the snout of a mouse, a few days later, we notice synapse loss in the somatosensory cortex. The microglia mediate the synapse loss via a chemokine receptor,” Schafer summarizes.
“A new area of focus for us is neurodegeneration – we’re moving into projects related to ALS, Alzheimer’s disease, and multiple sclerosis,” Schafer says, looking to the future. “We’ve only scratched the surface of what these cells do. It’s an exciting time for the field.”
View BioLegend's antibodies here. Also, we'd love to hear your antibody experiences - please consider leaving a 2-minute review for your chance to win a $400 Amazon voucher.
