Microplate reading technology for improved assay stability in drug discovery workflows
Explore the importance of stabilizing temperature in microplate reading technology to achieve robust and reproducible data in your drug discovery workflows
17 Mar 2023

Drug discovery and screening is a complex and time-consuming process that requires the use of advanced technology and specialized equipment to ensure data integrity and accuracy. Uniform and reproducible detection conditions are essential to ensure the reproducibility and accuracy of results, leading to more reliable data and ultimately facilitating successful research outputs. In this context, temperature is often overlooked.
In this article, Ann-Cathrin Volz, application specialist at BMG LABTECH, describes the key considerations to help optimize data quality and reproducibility in drug discovery. Plus, Volz discusses a novel microplate reading technology addition that provides assay stability through the control of external conditions such as temperature, and how this technology can help enhance the quality of the data and accelerate the drug discovery and development pipeline.
Maintaining steady temperature to ensure robust and reliable data
Achieving reproducible data in drug discovery can be challenging due to the complexity and variability of biological systems and experimental conditions. Standardizing protocols and controlling variables such as temperature and humidity are important steps in improving reproducibility. Ann-Cathrin Volz discusses why this is a significant challenge that drug discovery units are facing today.
“The hurdles that currently exist in drug screening are very diverse and complex,” Volz explains. “A basic requirement for all drug discovery programs is that the generated data are reliable and can be generated in a reproducible manner. Moreover, in today’s globalized landscape, it is required that the compound libraries present at different sites worldwide are dealt with in the same manner, and that data generated at these different sites are robust and comparable.” It is currently common practice to use the same kits, analytic devices, and protocols across units to ensure comparable conditions. But in addition to standardized operating procedures (SOPs), a steady experimental environment is fundamental for robust and high-quality data.
Volz explains that soft factors such as temperature and humidity are often overlooked. The climate varies between locations of drug discovery units worldwide, but temperature fluctuations within units are also an everyday occurrence in many laboratories. “These temperature fluctuations may derive from heating, air-conditioning, open windows, sunlight, seasonal changes, waste heat of other instruments, or from staffing,” Volz comments. “This can have a significant effect on pharmacological experiments like biochemical reactions or binding kinetics which are oftentimes evaluated on microplate readers.”
As many drug screening experiments rely on microplate readers for high-quality and reproducible data, it is necessary to consider all factors that may affect the instrument’s accuracy and precision. The influence of sample handling, such as pipetting accuracy, the choice of assay, and the use of standardized protocols play a major role. However, it is also important to ensure that samples are kept at a constant temperature.
“Typically, the temperature inside a plate reader is higher than the room temperature (RT),” says Volz. “This is due to the heat generated by the electronic components of the instrument during operation. For temperature-sensitive assays, preparing and measuring a microplate at different temperatures can be problematic. Due to the different temperatures, the samples on the plate can cool down or warm up after their transfer to the microplate reader. In temperature-dependent assays, this can affect the signal output, easily resulting in well-to-well variability or even in trends across the plate.”
When screening larger libraries, it is also important to measure at the same temperature over a prolonged time to avoid temperature-related effects on the data and ensure comparability among different plates in a campaign.
Counteracting temperature fluctuations with novel technology for microplate readers
While external temperature is a commonly overlooked consideration in drug discovery, there are also limitations in current microplate reader technology that can lead to inconsistent data due to temperature fluctuations. “Regular microplate readers can control the internal temperature from a few degrees above RT to 45°C or higher,” explains Volz. “This does not only mean that they cannot go below RT, but that they cannot reach it at all as the operation of the unit automatically causes a certain heating of the incubation chamber. Due to this and to the ever-present temperature fluctuations in laboratories, measurement under constant conditions is very hard to achieve.”
A new add-on for the PHERAstar® FSX microplate reader by BMG LABTECH has been designed to match room temperature conditions and ensure maintenance at any temperature between 18 and 45°C. The ‘Advanced Assay Stability’ (AAS) system achieves this by automatically heating or cooling the microplate measurement chamber depending on the external environment.
Volz describes how this solution ensures the microplate reader maintains a steady temperature that is unaffected by external environmental changes while in operation. “By setting the unit to RT, a temperature gradient between ambient temperature and the temperature in the reader is avoided. Consequently, plates can be prepared, incubated, and measured at the same temperature. Furthermore, the temperature can be kept constant over days and weeks, ensuring identical detection conditions for all plates in a batch or a screening campaign. This option is particularly beneficial for temperature-sensitive applications, kinetic measurements, or assays that need to be run at RT or below.”
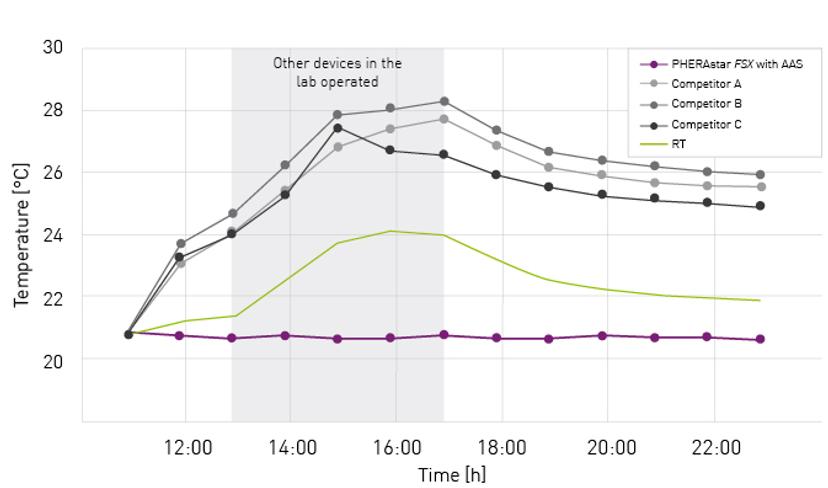
“Another advantage results from the special design of the PHERAstar FSX with AAS,” Volz continues. “The AAS is a closed system, which eliminates the external airflow to the inside of the plate reader. The limited airflow helps to keep humidity constant and reduce sample evaporation when compared to conventional plate readers.”

This system offers benefits for researchers running temperature-sensitive assays as well as kinetic measurements, and for all users with high data quality demands, particularly in screening facilities.
“Groups or collaboration partners who generate data in different geographic locations will increase reproducibility and comparability of their results,” Volz adds. “In addition, multi-user labs with different needs and applications may benefit from the AAS system since it enables a quick switch between applications at high and low temperatures.”
Volz summarizes, “the PHERAstar FSX with AAS provides improved assay stability and more reliable data which enable reproducible screening results across laboratories in different locations and environments worldwide.”

