MP Biomedicals and A*STAR co-develop rapid antibody point-of-care test kit for SARS-CoV-2
23 Jul 2020
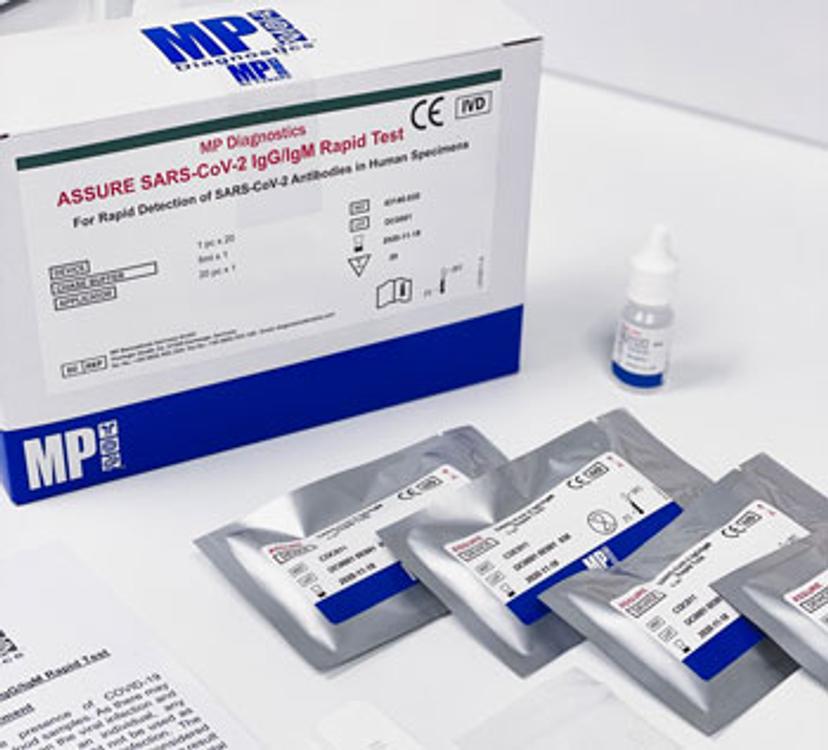
MP Biomedicals, a diagnostic corporation which has been operating for over 30 years focusing on various infectious disease testing development and manufacturing, has announced the successful development of a rapid antibody test kit for COVID-19, in collaboration with the Agency for Science, Technology and Research (A*STAR).
Named as the ASSURE® SARS-CoV-2 IgG/IgM rapid test, or ASSURE® in short, the test kit detects antibodies produced by the human immune system in response to exposure to SARS-CoV-2. It produces accurate results in as little as 15 minutes and employs a lateral flow format similar to those used in home pregnancy tests. ASSURE® was developed and manufactured in Singapore. It can be deployed at or near the point of patient care, and has been distributed to regions such as Europe, Africa and South America. MP Biomedicals intends to file for Emergency Use Authorization (EUA) from the US FDA for this product as well.
The test kit detects IgG and IgM antibodies, which are produced by the body to combat SARS-CoV-2, from samples of human blood, plasma or serum. Studies have shown that levels of IgG and IgM appear to be correlated with the severity of COVID-19, thus they are good biomarkers for confirming positive or past infection.
Aligned with the current recommendation by the World Health Organization, point-of-care or rapid serology tests including ASSURE® rapid antibody test kit should not be used in the clinical diagnosis of COVID-19 infections or in the evaluation of persons with acute respiratory symptoms, especially within the first 14 days of illness. This is to avoid giving patients false reassurance that they do not have the infection, arising from a negative result. However, ASSURE® rapid antibody test kit can help to determine whether an individual has been previously exposed to the virus and generated antibodies as a result. This can help identify asymptomatic individuals or those with only mild symptoms who were not subjected to RT-PCR testing.
The technology behind the ASSURE® rapid antibody test kit utilizes proprietary synthetic SARS-CoV-2 proteins. These proteins bind to the IgG and IgM antibodies if the antibodies are present in the specimen samples. MP Biomedicals used it to develop the product based on their lateral flow platform. The Diagnostics Development (DxD) Hub, a national platform hosted by A*STAR's commercialization arm, A*ccelerate, co-developed the validation protocols and quality controls. The ASSURE® rapid antibody test kit was evaluated by the National University Hospital's (NUH) Department of Laboratory Medicine and demonstrated good results for both serum and whole blood. The sensitivity of the kit performed well as compared to commercial immunoassays, when tested with convalescent blood from recovered COVID-19 patients in the clinic.
The ASSURE® rapid antibody test kit has been granted Provisional Authorization by the Health Sciences Authority (HSA) for its intended use in Singapore.
Dr Delynn Xu, Senior R&D Manager, MP Biomedicals, said: "The development and manufacture of ASSURE® is a successful collaboration between MP Biomedicals and A*STAR through tremendous joint work. We are not the first one in the market but chasing the best performance is always our primary goal. With this rapid antibody test kit, we are proud to contribute to the global fight against COVID-19."
"We identified a human monoclonal antibody which binds to SARS-CoV-2, and it proved to be useful during the early development phase of this rapid test kit," said Associate Professor Tan Yee Joo, Joint Senior Principal Investigator, Institute of Molecular & Cell Biology (IMCB), A*STAR, and National University of Singapore's Yong Loo Lin School of Medicine. "IMCB is glad to assist our industry partner in their COVID-19 product development," she added.
Dr Sidney Yee, Chief Executive Officer, DxD Hub, A*STAR, said: "It is absolutely critical that we continue to transfer R&D know-how to biotech companies, to scale up and let more labs tap on this diagnostics test kit to screen patients. This rapid serological point-of-care test kit for COVID-19 by MP Biomedicals and A*STAR will complement global efforts to develop more efficient diagnostics, as the COVID-19 situation continues to evolve."
Mr Ng Boon Heong, Chief Executive Officer, Temasek Foundation, said: "We seek to actively pilot innovative solutions for community care. This project is an opportunity for us to work together with companies with a local presence, such as MP Biomedicals; R&D institutions such as A*STAR; medical partners such as NUH to enable development and access to point of care rapid serological test kits. These kits help identify segments of our communities that are recovering from or previously infected by SARS-CoV-2 to ensure their safe return back to work. This is one of the cases where we placed an initial commitment to help MP Biomedicals scale up their production locally, and piloted its use in our local community."
For more of the latest science news, straight to your inbox, become a member of SelectScience for free today>>
