New Study Reinforces Major Advantages of Dako's HER2 IQFISH pharmDx™ in Breast Cancer Diagnostics
21 Feb 2014
Dako, an Agilent Technologies company and worldwide provider of cancer diagnostics, today announced positive findings in an independent French multicenter study on the performance of Dako's HER2 IQFISH pharmDx™.
The Toulouse, France, breast cancer group led by Magali Lacroix-Triki, has published a peer-reviewed publication in the Journal of Histopathology(1) that supports the use and performance of Dako's HER2 IQFISH pharmDx™ for HER2 gene amplification status assessment in breast cancer. Dako introduced the cutting-edge IQFISH hybridization buffer chemistry in 2012. The IQFISH buffer reduces test turnaround time from 17 hours to just 3.5 hours.
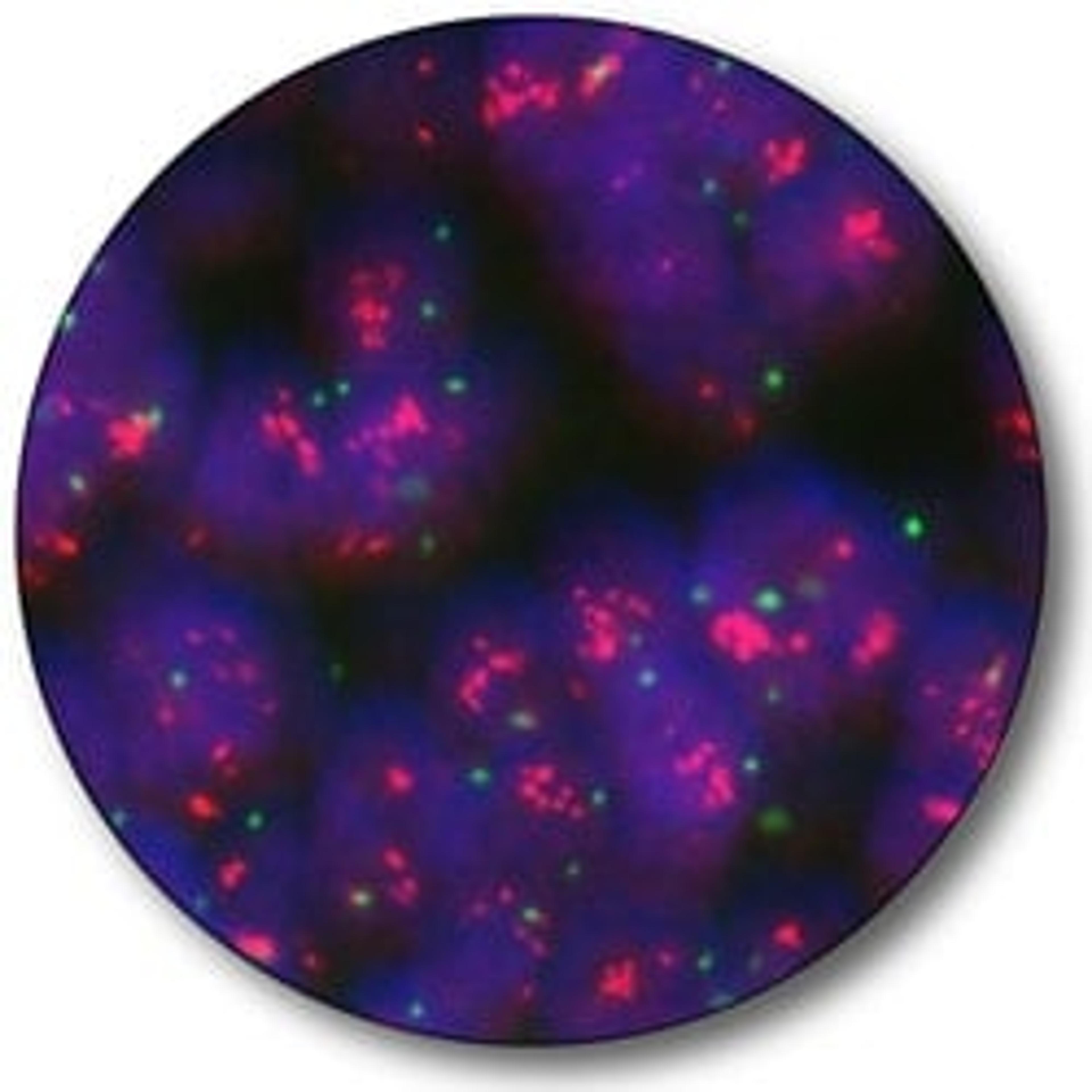
Quantitatively determination of HER2
More than 1.5 million new cases of breast cancer are diagnosed each year worldwide (2). As an aid in the treatment decision, HER2 IQFISH pharmDx™ can quantitatively determine HER2 gene amplification in the tumor.
The French study found that Instant Quality (IQ) FISH provides "excellent quality signals without any background staining, thus allowing excellent reading conditions(1)." The study also points out "almost perfect agreement between IQFISH and FISH" and that IQFISH is a "safe, reliable and fast method that is easy to use in routine practice" with few reruns. The statement is significant in that the tissue came from five different laboratories using four different fixation methods.
IQFISH a useful alternative to FISH
The study concludes that the highly concordant results (99.3%) and assay robustness support IQFISH as a useful alternative to FISH, allowing reliable assessment of HER2 status. It also finds that using this method enables pathologists to report the results to the oncologist within a day, meaning patients can begin cancer treatment sooner than ever.
The study's findings follow those of another study showing major advantages of using Dako's IQFISH chemistry. A Hungarian study, published in 2013 in the Journal of Applied Immunohistochemistry and Molecular Morphology(3), showed several advantages of the new hybridization buffer:
"The one-day HER2 IQFISH approach is a potent method for the determination of HER2 status offering some relevant advances further to the short incubation time, including enhanced tissue integrity and preserved fluorescent signal intensity when compared with the conventional HER2 FISH kit." Hegyi et al., 2013(3).
"At Dako, we are focused on the advancement of cancer diagnostics, so we are particularly proud of these findings regarding our IQFISH buffer," said Britt Meelby Jensen, Dako general manager and vice president. "We understand that the speed and accuracy of patient sample processing and the quality of test performance affect the treatment decisions and outcome for patients."
Additional benefits of IQFISH
An additional benefit of the IQFISH buffer is that it avoids the use of toxic formamide in assays and eliminates concerns for pregnant laboratory technicians. The buffer is environmentally friendly and does not require a fume hood during use(4).
References:
(1)Franchet C, Filleron T, Cayre A, Mounié E, Penault-Llorca F, Jacquemier J, Macgrogan G, Arnould L, Lacroix-Triki M. Instant-quality fluorescence in-situ hybridization as a new tool for HER2 testing in breast cancer: a comparative study. Histopathology 2014 Jan;64(2):274-83.
(2)Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray, F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed on 24/January/2014.
(3)Hegyi K, Lønborg C, Mónus A, Méhes G. One-day FISH approach for the high-speed determination of HER2 gene copy status in breast carcinoma. Appl Immunohistochem Mol Morphol 2013 Dec;21(6):567-71.
(4)Matthiesen, S. H. and C. M. Hansen. Fast and non-toxic in situ hybridization without blocking of repetitive sequences. PLoS One 2012; 7:1-7 (e40675).