NEW SynStats Software for Antibiotic and Vaccine Developers
3 Oct 2013
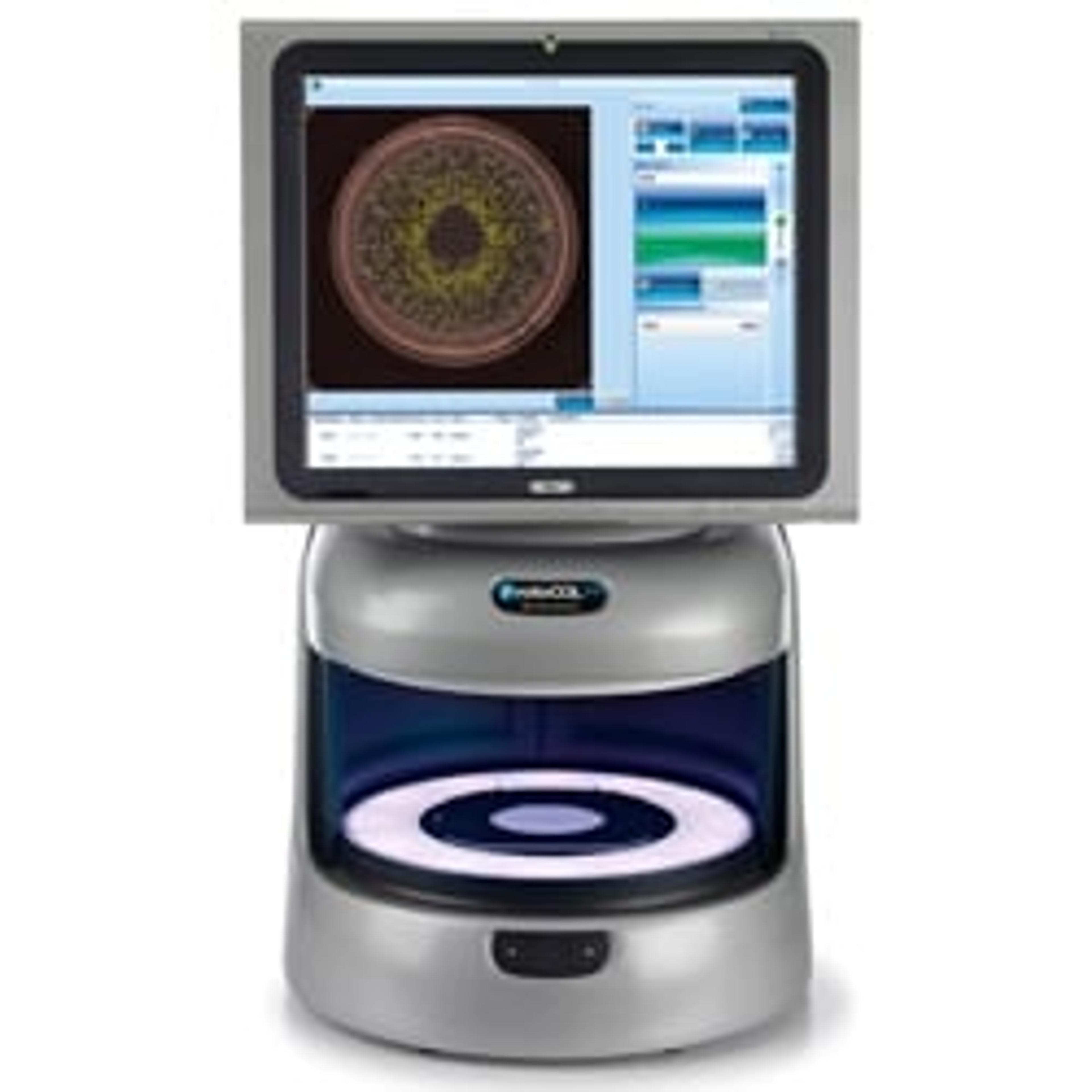
Scientific Digital Imaging’s [SDI’s] Synbiosis Division, a world-leading manufacturer of automated microbiological systems, today introduced its SynStats statistical analysis software, designed to rapidly analyse data from the ProtoCOL 3 automated colony counter and zone measurement system. The software provides automatic antibiotic efficacy and vaccine potency results from raw data, significantly increasing productivity of testing.
Using SynStats cost-effective analysis software, with just one click, microbiologists can export their zone measurement and colony count data from the ProtoCOL 3 directly to SynStats. The software then quickly and easily provides a range of analyses including analysis of assays with single or multiple Latin Squares; producing a 5 + 1 standard curve and 2 + 2 and 3 + 3 Petri dish assays; high-lighting outliers and replacing missing values; performing parallel line and slope ratio analysis and producing potencies with confidence limits, as well as providing analysis of variance. Results are provided in graphs and tables, thus saving time with transferring data and presenting the results in a regulatory compliant format.
SynStats, is both European Pharmacopoeia and US Pharmacopeia compatible and can be supplied with installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) documentation to allow the software to be integrated with the ProtoCOL 3 in a Good Manufacturing Practice (GMP) compliant environment. These features make SynStats ideal for ProtoCOL 3 users developing new antibiotics or vaccines that need to securely archive and present all their results to regulatory authorities such as the US FDA (Food and Drug Administration) and the EMEA (European Medicines Agency).
Kate George of SDI’s Synbiosis Division explained: “Microbiologists producing data to measure antibiotic effectiveness or vaccine potency often have large numbers of Excel spreadsheets containing data to transfer and analyse. This can be a time consuming and potentially risk prone activity because the figures do not always transfer across directly into statistical analysis packages and can require additional manual manipulation. What they need is analysis software which is compliant with US and European regulations that they can input raw zone measurement or colony count numbers into and easily obtain vaccine or antibiotic efficacy results out from, without them having to become statistics experts.”
Kate added: “Our ProtoCOL 3 design team has responded to this need with extensive development work. We believe that the resulting powerful software will significantly de-risk the data transfer, as well as increase throughput of antibiotic and vaccine testing in any pharma or biotech which chooses to integrate the SynStats analysis software with their ProtoCOL 3 automated technology.”