Next-Generation Immunotherapy: Combining Virus-Specific T Cells with Other Immuno-Oncology Technologies to Treat Solid Tumors
Dr. John Connolly, CSO of Tessa Therapeutics, reveals how a new approach to cell-based therapy in cancer could circumvent severe immunotherapy side effects and effectively target aggressive solid tumors
15 Aug 2019

Immunotherapy holds great potential for potent and durable anti-cancer responses. Unlike chemotherapy, modern immunotherapies such as checkpoint inhibitors have shown anti-tumor efficacy in otherwise untreatable lung and kidney cancers, malignant melanoma, and other advanced cancers. However, despite the promise of immunotherapy, its efficacy has only been realized in a subset of patients.
Now, a novel, rational approach to immunotherapy – involving the selective expansion and infusion of virus-specific T cells (VSTs) – shows potential in mediating the same powerful attack on aggressive tumours whilst not being plagued by some common side effects of conventional T cell therapy, such as cytokine storm. This often-deadly event is characterized by an overproduction of immune cells and their activating compounds, that can result in respiratory distress and multiple organ failure.
In an exclusive interview with Dr. John Connolly, Chief Scientific Officer of Tessa Therapeutics, SelectScience finds out how these VSTs could provide new hope for a wide spectrum of aggressive tumors.
The promise of immunotherapy as an effective tool in oncology
Throughout the 20th century, chemo and radiotherapies were the mainstay treatment regimens for cancer patients. Designed to ablate proliferating cells in the body, we’ve seen partial success with combination approaches in haematological malignancies and some solid tumors.
It wasn’t until the 1990s that the era of personalized therapy begun. With targeted treatments such as Gleevec entering the market, the field saw a resurgence in efforts to find cancer’s Achilles’ heel. Modern day genomic technologies have since opened the gateway to more effective rational drug design and, as such, many hundreds of molecularly-targeted compounds now exist in the oncologist’s toolbox.
Yet, as Dr. Connolly explains, the heterogeneity of the disease means that no current standard of curative therapy exists and that is why harnessing the dynamic nature of the immune system for cancer therapy is an exciting concept.
“Although small molecules work incredibly well in a few patients, most don’t respond at all. The development of anti-CTLA4 and anti-PD1 immunotherapies were revolutionary and was able to produce excellent results in patients with primary tumors of different origin.”
Immune cells originate in the bone marrow and are constantly being replenished from dividing adult stem cells. For decades, we’ve been weakening the most effective anti-cancer tool, the immune system, through the chemotherapy agents being administered to patients in the first place.
The immune system plays a significant role in the immunosurveillance and elimination of tumors.
Connolly talks us through the journey toward this realization: “Initially, it was thought that the immune system was simply a self, non-self, filter. But we now know that it’s also involved in destroying cells with aberrant metabolism and genetic mutations. This became clear when treating transplant patients with immunosuppressive drugs. These individuals had a greater cancer occurrence rate, indicating the immune system plays a significant role in the immunosurveillance and elimination of tumors. A select class of drugs called checkpoint inhibitors leverages these surveillance mechanisms to eliminate tumors. This revolutionary therapy has shown fantastic results in very late stage tumors”.
However, only a subset of patients responds to checkpoint inhibitors. A new class of therapy called CAR-T has emerged which takes the patient’s own cells, modifies them and directs them against the tumor. In 2014, the FDA granted breakthrough designation status to CD19-directed CAR-T cells, following a paradigm-changing clinical trial in adults with acute lymphoblastic leukemia (ALL). In 2017, CAR-T was officially approved for use in children and adults with ALL.
“We’re now at a really exciting stage of refining and rationalizing our immunotherapy design. It’s a hugely exciting time to be in this field of research,” says Connolly.

Tessa’s virus-specific T cell technology: evidence of minimal toxicity and maximum anti-tumor potency
There are now multiple types of cell therapies being trialled across the world, of which CAR-T is one of the first to be approved for commercial use. Other immunotherapies include adoptive cell transfer, as well as oncolytic viruses and cancer-based vaccines.
Explaining where Tessa Therapeutics fits in, Connolly adds: “Tessa has taken a completely new approach to cell therapy for solid tumors. CAR-T therapy involves the random expansion of all T cells isolated from the patient, which manifests a mixed release of anti-inflammatory compounds that is most likely to induce cytokine release syndrome, otherwise known as the ‘cytokine storm’.”
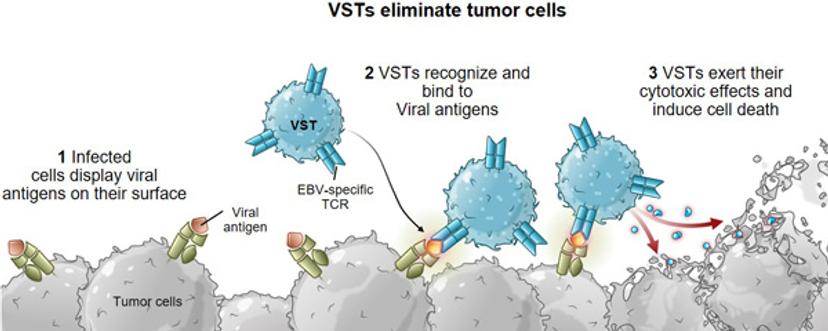
“Through Tessa’s combined research efforts with our academic collaborators, however, we’ve learned that virus-specific T cells (VSTs) are the most tuned to cancer cell killing. They have the ability to recognize and kill infected cells while activating other parts of our body’s immune system for a coordinated response. Previous VST clinical trials have demonstrated the long-term, potent anti-tumor activity of VSTs without inducing severe toxicity.”
Virus Specific T cells (VSTs) have the ability to recognize and kill infected cells while activating other parts of our body's immune system for a coordinated response.
“Additionally, these unique properties of VSTs make them an ideal candidate for allogeneic therapy as well. The virus specificity of the T cell means there is low risk of graft versus host disease (GvHD). We believe the combination of VSTs with CAR targeting technology, or the cell lysing capability of an oncolytic virus, have the potential to further increase the efficacy of our platform. ”
Tessa has several exciting product candidates in their clinical and pre-clinical autologous programs that target a wide range of solid tumors including nasopharyngeal carcinoma, cervical cancer, lung cancer, breast cancer, bladder cancer, as well as head and neck cancer. Tessa’s most advanced VST therapy in clinical development is currently in an ongoing multi-center Phase 3 trial for nasopharyngeal cancer, recruiting 330 patients across 5 countries. In addition, the company is leveraging its platform to develop an allogeneic therapy to address Epstein-Barr virus (EBV)-associated lymphomas.
Tackling aggressive brain tumors through a novel multi-CAR VST approach
Another novel product candidate which the company has begun to explore in collaboration with leading US paediatric hospital, St Jude Children’s Research Hospital, is a multi-CAR VST approach to target paediatric high-grade gliomas, an aggressive form of brain cancer in children which currently has very few treatment options.
The multi-CAR VST approach targets multiple tumor antigens expressed in paediatric gliomas, which will help address the problem of resistance through antigen loss, a common escape strategy of cancer. The therapy is currently under pre-clinical development and if successful, could be adapted to other paediatric cancers that cannot be cured with conventional therapies.
VSTs in combination with other immuno-oncology agents
With a body of evidence to suggest VST cell therapy can replicate the anti-viral response in vivo to eliminate tumor cells, Tessa is making waves in the field of immunotherapy. Dr. Connolly believes its technology could truly transform advanced tumor prognoses and the lives of many.
At this point, the company is still in the process of refining its antigen combinations using some of the most innovative in vitro assays. The highly-adaptable nature of these tumors to therapy necessitates a multiplex antigen approach. Connolly explains how the xCELLigence® Real-Time Cell Analysis Technology, by ACEA Biosciences - a part of Agilent Technologies, enables his team to simultaneously decipher the killing effect of multiple antigens.

“This technology enables the analysis of around 100 different experimental modifications to our cells, using multiple therapeutic combinations. We can do all of this with very few cells, in a label-free manner. What’s also particularly pertinent to our research is the ability to collect and analyze the supernatant afterwards. We want to understand how our VSTs mediate downstream immune events, and this allows us to do that.”
Looking to the future, Dr. Connolly has visions to couple up the VST technology — which activates the adaptive anti-viral response — with other rational combinations that stimulate the body’s innate immune response.
Already within Tessa’s fast-growing pipeline, there is an ongoing investigator-sponsored Phase 1 trial evaluating the safety and optimal dose selection of armored HPVSTs in combination with another anti-PD-1 antibody.
Tessa has also made progress in the pre-clinical development of a first-of-its kind combination approach that integrates cell therapy, oncolytic virus and checkpoint inhibitors into one therapy - Human Epidermal Growth Factor Receptor 2 (HER2)-CAR VSTs + Oncolytic and Helper-Dependent Adenovirus, targeting HER2-positive solid tumors. Tessa is now working on initiating a Phase I trial with Baylor College of Medicine.
“The field of cancer immunotherapy is going to see real advances in combination cell therapies in the coming years. Tessa is very excited to be pursuing this new frontier to try and find cutting edge next-generation therapies that hold promise of treating patients more effectively.”
Dr. John Connolly presented this work in ACEA Biosciences’ 7th Annual Cancer and Immunotherapy symposium, in Atlanta, on March 30. Watch his session titled “Virus Specific T cells as a Platform for Solid Tumor Immunotherapy” here>>
Visit the event page for more information here.

