QIAGEN Demonstrates Expanded Bioinformatics Workflows at ASHG
20 Oct 2014
QIAGEN N.V announced a number of new capabilities for its Ingenuity® Variant Analysis™ and CLC Cancer Research Workbench solutions that accelerate the workflows for researchers to move from sample to insight with next-generation sequencing (NGS). The company is demonstrating the latest workflows for analysis and interpretation of NGS data, focused on hereditary disease and detection of somatic driver mutations in cancer, at the American Society of Human Genetics (ASHG) annual meeting from October 18-22 in San Diego, California.
“Adoption of QIAGEN’s universal bioinformatics solutions is growing rapidly as we continue to integrate and expand the capabilities of our applications and knowledge bases from CLC bio, Ingenuity and BIOBASE. Thousands of researchers to date have already analyzed over 250,000 human DNA samples using QIAGEN bioinformatics products,” said Dr. Laura Furmanski, Senior Vice President of QIAGEN and head of the Bioinformatics Business Area. “Data analysis and interpretation remain a significant bottleneck in next-generation sequencing. We are increasing QIAGEN’s leadership in bioinformatics by further integrating and expanding our portfolio of universal solutions to enable disease-focused researchers to move rapidly from raw data to valuable insights. We are pleased to demonstrate our streamlined, advanced analysis workflows for translational research, other scientific fields and clinical applications to the human genetics community at ASHG.”
Ingenuity Variant Analysis integrates BIOBASE HGMD
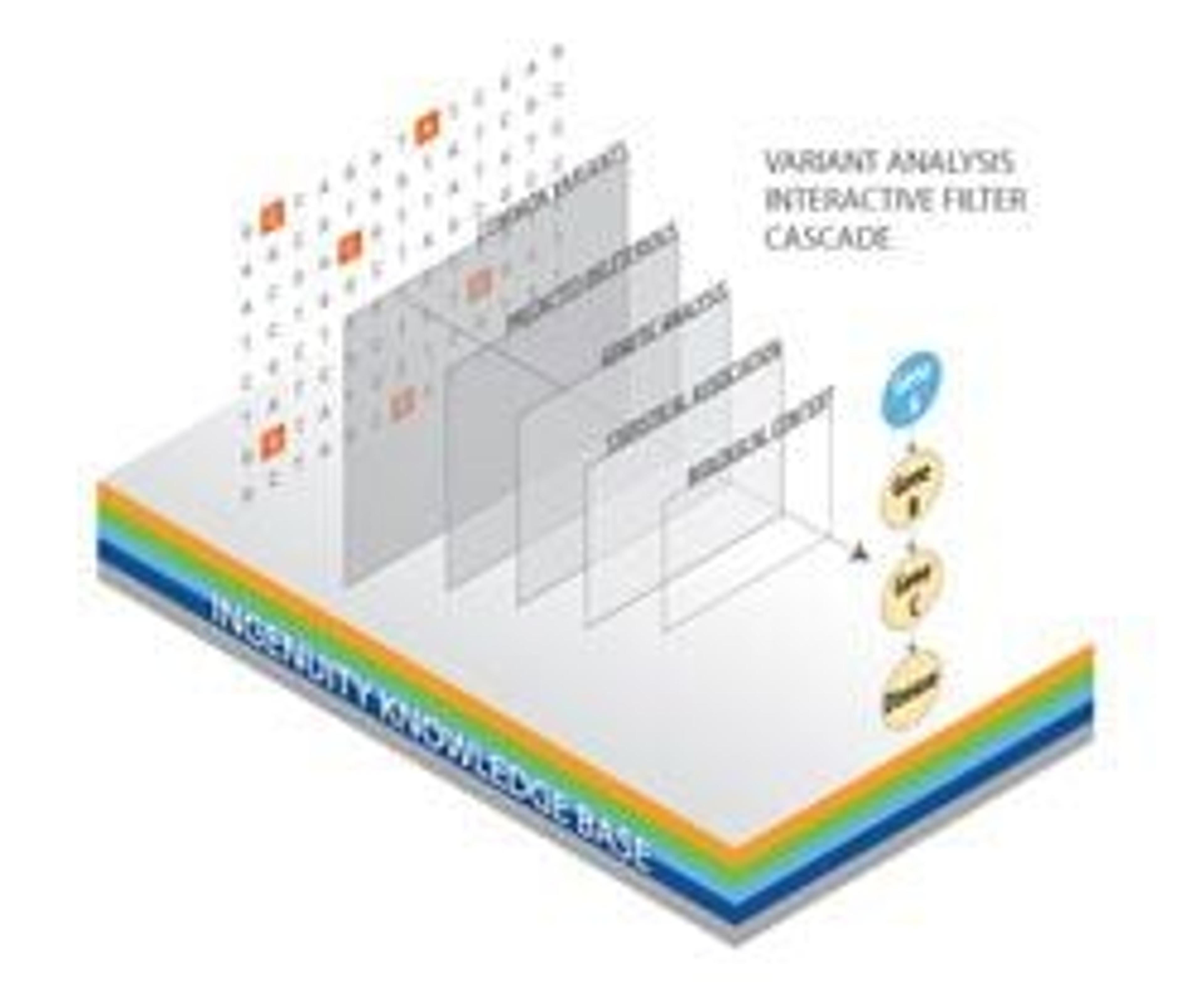
QIAGEN’s Ingenuity Variant Analysis is a web-based software application that quickly filters genetic variants in a secure, private cloud-based environment to identify variants most likely to cause disease. Ingenuity applications provide researchers a powerful platform to efficiently evaluate data generated by high-throughput NGS technologies. Ingenuity Variant Analysis leverages the Ingenuity Knowledge Base, a repository of expertly curated biological interactions and functional annotations created from millions of individually modeled relationships between proteins, genes, complexes, cells, tissues, drugs, and diseases. To support users focused on hereditary disease, QIAGEN now has fully integrated HGMD Professional with Ingenuity Variant Analysis so that researchers no longer require a separate HGMD license. HGMD is a unique resource that provides comprehensive data on human inherited disease mutations and is widely used in human genetics research, diagnostics and personal genomics applications. Through this integration, users can now utilize ethnicity inference to simplify dataset groupings and to identify variants associated with target traits (such as high cholesterol or physical traits) at no additional cost.
CLC Cancer Research Workbench adds CNV detection and integration with Ingenuity Variant Analysis
QIAGEN’s CLC Cancer Research Workbench, the first comprehensive, user-friendly and customizable cancer-focused informatics solution, provides scientists and clinicians with tools to discover prognostic markers, identify subclonal somatic mutations, detect inherited traits, find biomarkers for drug response, and determine new oncogenes. All results can be filtered, visualized and compared with relevant databases. Users will now also be able to detect copy number variations (CNVs) and variants from RNA-seq data, which can be further analyzed using Ingenuity Variant Analysis for causal variant identification. QIAGEN has also developed the first “FastQ-to-insight solution,” which integrates CLC Cancer Research Workbench directly with Ingenuity Variant Analysis. This new Workbench plug-in allows users to identify and interpret somatic cancer driver mutations with one click using Ingenuity Variant Analysis and to visualize the results in both products to identify and validate the best candidates. A demonstration of this new tool will be shown at ASHG. The product’s launch is expected at the end of October.
QIAGEN Presence at ASHG
QIAGEN scientists will be discussing Ingenuity and CLC bio solutions in the company’s booth (#936-938) throughout the meeting and also during an Exhibitor Education Event in Room 11A at the Convention Center on Tuesday Oct. 21 from 12:30-1:45 p.m. In addition, more than 30 posters will highlight the use of Ingenuity and CLC bio products, including:
Saturday, Oct. 18, HGVS meeting (preconference of ASHG)
• Comparison and interpretation of variants in RNA and DNA from sarcoma cancer sample
Sunday, Oct. 19, 5-6 p.m.
• Comparing variant filters from transcriptome and exome sequencing data (#1578S)
• Exome sequencing of multiplex oral clefts families detects recurrent shared rare variants in nine genes (#742S)
Tuesday, Oct. 21, 2-3 p.m.
• Identification of differentially-expressed genes and somatic mutations in esophageal adenocarcinoma cancer patients (# 1391T)