Redefining quality in cell line development
From monoclonality to droplet-based technology, find out the key to cell line development success
9 Nov 2023

A robust cell line development process is pivotal for the large-scale production of biopharmaceuticals, therapeutic proteins, monoclonal antibodies, vaccines, and industrial enzymes. The primary aim of cell line development is to produce stable and high-producing cell lines to facilitate consistent generation of these products, driving medical treatments, research advancements, and industrial processes.
In this exclusive interview, we speak with Richard Hammond, Chief Technology Officer, Sphere Fluidics about the key technology driving quality and efficiency in cell line development. Hammond discusses how advanced technologies and measurement-based decision making are changing the approach to regulatory compliance. He also explains the benefits of droplet-based technology for workflow efficiency and cell viability and how interdisciplinary partnerships cultivate innovation.
Quality in cell line development: An industry standard
When it comes to cell line development, meeting the rigorous requirements set by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) poses a complex problem that any developer must solve. These regulatory bodies are responsible for overseeing the development and approval of biopharmaceutical products. From ensuring the safety and efficacy of therapeutic proteins to assessing the quality and consistency of cell lines, complying with regulatory guidelines is essential.
Regulatory authorities expect the use of state-of-the-art techniques.
Richard Hammond Sphere Fluidics
One key factor to ensuring compliance with regulatory standards in cell line development is to demonstrate monoclonality, or a single-cell progenitor. Hammond comments, “this is a regulatory requirement, it is not optional. Both the U.S. FDA and the EMA explicitly state the need for monoclonality in their guidelines, such as the ICH Q5D.” Monoclonality is crucial for ensuring safe and effective products and maintaining overall quality control. Hammond explains, “biology’s inherent heterogeneity necessitates a thorough understanding of the starting point of a cell line to monitor and control product quality.”
For the FDA and EMA, proving that products are safe and effective is key. Hammond discusses the evolving face of regulatory compliance in response to advanced technologies. “Regulatory authorities expect the use of state-of-the-art techniques. This drives developers to continually explore novel approaches for assessment and seek the most current tools and techniques to deliver the highest standards of performance in their field.”
The continuous advancement of analytical technologies enables us to make more informed decisions based on better data. “At any point in time, we are limited to working with the tools available to us,” elucidates Hammond. “As technology constantly evolves, there is an expectation, especially from regulatory perspectives, to leverage new advancements.”
New technologies empower scientists to gain deeper insights into cell analysis, examining individual cells with heightened resolution. The ability to assess various aspects such as phenotype, proteome, transcriptome, and genome, yields richer and more comprehensive data. Hammond comments, “This improved data quality enables us to make better-informed decisions, whether in research and development or manufacturing quality assurance. By striving for higher resolution, improved data, and refined decision-making, we continuously advance the underlying concept of quality, efficacy, and safety in cell line development.”
Cost and contingency: Striking the balance
Biopharmaceutical development is a costly process, making it essential for developers to optimize workflows to maximize success rates. A key focus is placed around generating a high titer cell line, that produces a high yield of the protein or antibody of interest.
Hammond explains, “The cost of developing and producing biologics is substantial, with fixed costs that require significant investment. If a cell line yields a low titer, the return on investment diminishes as the output is small relative to the costs incurred, especially when considering the additional expenses of quality control processes.” Hammond continues, “in contrast, high titers offer economic advantages by maximizing the output for the same fixed cost, making the initial investment worthwhile.”
A crucial aspect of this cost optimization is the early selection of high-producing cells from a large pool. Hammond describes how this can have a substantial impact on enhanced productivity, cost-effectiveness, and shorter development timelines, ultimately leading to more successful and efficient biopharmaceutical production. “To minimize fixed costs, it is crucial to identify a high-producing clone quickly and efficiently. By screening a wide range of potential candidates early on, the probability of finding the desired high producer increases,” Hammond explains. “This approach allows researchers to focus their efforts and resources on the most promising candidates, reducing time spent on less productive options.”
The selection of high-producing cells is driven by the interplay of economics and probability. Maximizing titers aligns with economic efficiency, while efficient screening and selection processes enhance the probability of identifying high producers. By striking the right balance, businesses can justify their investments by delivering high-value biopharmaceutical products while minimizing unnecessary costs.
Technology integration: Working in harmony
The astounding advancement of technologies in the last few decades has sparked revolutionary transformations in cell line development and beyond. “Technology has become an essential cornerstone in modern industry, shaping the way people work and enabling the development of groundbreaking products that were previously unimaginable,” Hammond says. The breadth of technologies involved in developing cell lines is extensive, ranging from biology and biochemistry-based techniques like sequencing, cloning, and editing, to physics and engineering technologies such as optics, fluidics, and thermodynamics. Hammond explains, “cell line development is utterly reliant on all these technologies working together and harmonized in very effective ways.”
An example of successful technology integration is the Cyto-Mine® platform by Sphere Fluidics. Cyto-Mine is a fully integrated platform for high-throughput single cell analysis, leveraging microfluidic picodroplet technology. Hammond explains, “each tiny droplet, with dimensions on the scale of tens of microns across, forms an emulsion of water droplets in oil. Each drop is comparable to a minute reaction chamber, but we can make millions of them.” By encapsulating single cells in these separate picolitre volume droplets, Cyto-Mine enables the rapid analysis (based on secreted or surface biomolecules), selective screening, sorting, imaging, and dispensing of single cells into individual wells of microtiter plates.
Integrated technology such as this can increase the efficiency of daily laboratory processes. Hammond explains, “Cyto-Mine offers hands-off automation, enabling scientists to work faster, more efficiently, and at a lower cost due to reduced reagent and plastic consumption. This yields immediate economic benefits in terms of speed, cost-effectiveness, and increased productivity.” Hammond adds, “this approach helps to address those fundamental economic challenges by increasing the probability of finding optimal cells early on from a large population.”
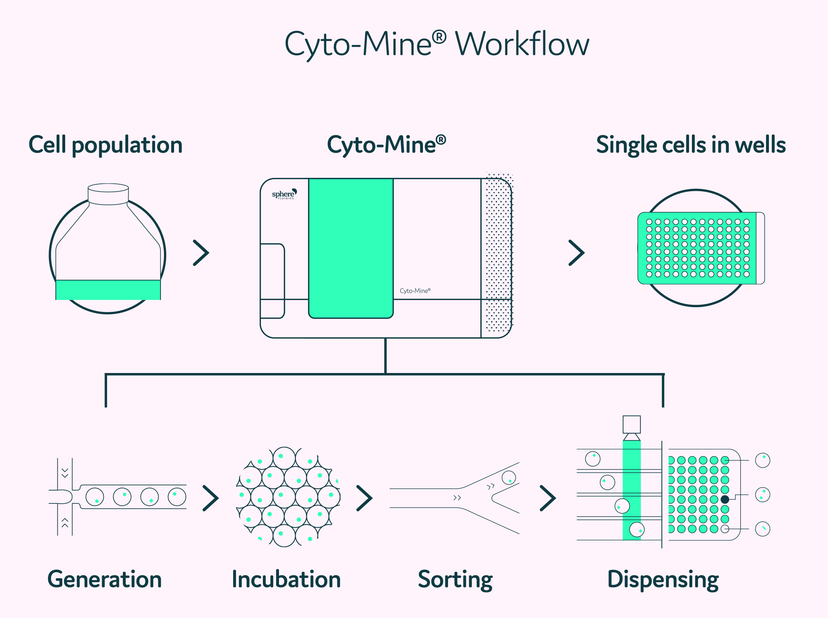
Cyto-Mine workflow for cell line development
Cell viability: Raising the bar with droplet-based technology
Not only does droplet-based technology address the core economic and probability challenges of in cell line development but provides a solution to ensure cell viability. Viability is a key consideration in cell line development to safeguard the integrity and optimal performance of the most promising cell line. Hammond explains, “microfluidics poses a significant challenge in cell line development due to the need for high-speed processing, which subjects the cells to considerable shear stresses. This can have adverse effects on cell viability and overall success, particularly with traditional flow cytometry systems.” Compartmentalizing the cells in picodroplets protects the cell, reducing the shear stress the cell is exposed to. Hammond says, “By encapsulating the cells within droplets, we provide insulation that shields them from shear stresses, ensuring their protection while achieving high processing speeds.”
The droplets are also compatible with a wide range of media, meaning cells can be maintained in familiar and optimal growth conditions. Moreover, Hammond details how the oil used in Sphere Fluidics’ solutions has been carefully designed to have a very high gas permeability, enabling the cells to engage in vital respiration and maintain crucial gas exchange processes necessary for their survival. Hammond says, “this combination of accommodating various media forms, facilitating gas exchange, and ensuring cell protection results in improved cell viability throughout the process. In addition to the benefits of cell-by-cell assessment and hands-off operation, this approach prioritizes cell well-being and enhances the overall outcome.”
Unlocking innovation: The power of partnership
Partnerships are fundamental to deliver transformative solutions.
Richard Hammond Sphere Fluidics
In the field of cell line development, innovation thrives on the integration of specialized knowledge and expertise. Hammond emphasizes the significance of partnerships between technology companies and biopharma laboratories. “As technology continually evolves and new advancements emerge, the partnership model remains essential.
Specialization is key in mastering specific techniques, such as FRET assays or AI software for image analysis. However, integration is equally crucial,” Hammond says. “This is where partnerships come into play, combining specialized expertise to achieve remarkable outcomes. These partnerships are fundamental to harnessing technology, depth of understanding, and the interdisciplinary integration required to deliver transformative solutions.” Sphere Fluidics serves as a prime example, excelling in assays involving droplets and microfluidics. By fostering partnerships between technology companies and biopharma labs, laboratories can enhance their workflows, optimizing the quality, safety, and efficacy of the final product.

