Repurposing Existing Drugs to Speed Up the Development of Therapies for Rare Muscular Dystrophies
Discover how a research group at the University of Geneva, Switzerland, is using an innovative approach to accelerate therapeutics through the drug development process
2 Jul 2017

Prof. Leonardo Scapozza and Dr. Hesham Ismail, of the Pharmaceutical Biochemistry Group at the School of Pharmaceutical Sciences of the University of Geneva, told SelectScience how they are using microplate reader technology to assist the process of repurposing existing drugs to treat rare diseases, such as Duchenne muscular dystrophy.
Prof. Scapozza’s research group brings together scientists with a broad knowledge base in pharmacology, structural biology, medicinal chemistry and molecular modelling in order to develop therapeutics in a variety of applications, including cancer, infectious disease and rare disease.
Dr. Hesham Ismail and Dr. Olivier Dorchies, a post-doctoral researcher and senior research collaborator working under Prof. Scapozza, focused their research on pharmacotherapy of muscle diseases.
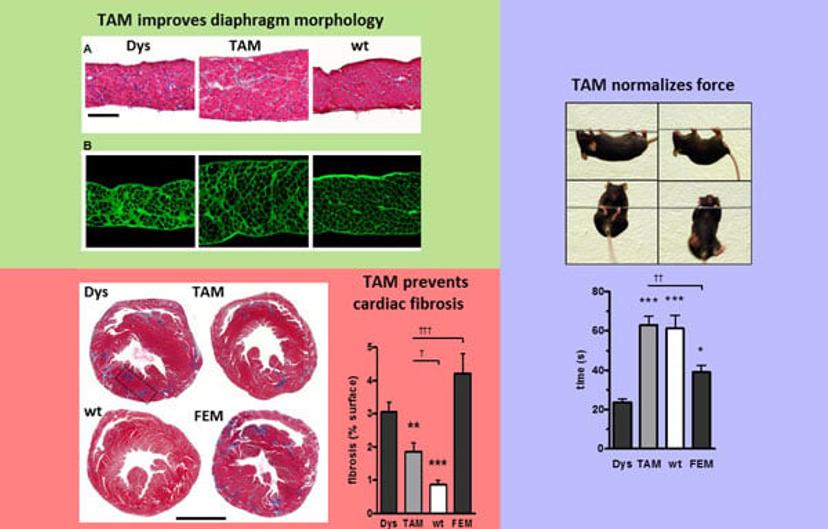
One of the key achievements of the group in this area was to provide preclinical data on drug efficacy and safety for three drugs for Duchenne Muscular Dystrophy – a life-limiting, progressive muscular disorder which affects around 1 in 3,500 males – fostering the launch of three clinical trials. The most recent example is tamoxifen, a drug that was first developed by AstraZeneca as a treatment for breast cancer that is entering a randomized placebo controlled phase 2 clinical trial.
There is no rational cure of diseases without knowledge of what happens on a molecular level.
Prof. Scapozza
SS: What is your approach to drug development?
Prof. Scapozza: ‘There is no rational cure of diseases without knowledge of what happens on a molecular level’ is the moto of the group. We use a multidisciplinary approach; a combination biochemistry, biophysics, chemistry and computational chemistry and molecular modelling. Since 2012 we have also added in the capacity to assess in vivo activity, by bringing in Drs Olivier Dorchies and Hesham Ismail, allowing us to have in vivo preclinical proof of concept of newly developed therapeutics.
SS: What are the advantages of repurposing existing drugs?
Dr. Ismail: We always work with the notion that patients suffering from severe muscle diseases such as Duchenne patients, don’t have the luxury to wait for a therapy to be developed from scratch, which can take between be 10 to 15 years. However, by repurposing existing drugs, we make a leap from 5 to 10 years in the development process. In the case of a recent industrial collaborative project, we began evaluating the pharmacological effects of the compound as a therapy for Duchenne patients in 2013 and it went into clinical trials just 18 months later.
Prof. Scapozza: The reasons why the repurposing process is faster is that all the toxicity and safety knowledge has already been produced from previous first-in man clinical phase I trials. So, to repurpose the drug, all you need to do is to prove efficacy in the pre-clinical model at doses within the therapeutic windows of the drug. In this case, the drug can be proposed to enter clinical trials. The Orphan Drug designation from the European Medicines Agency for repurposed drugs can help getting these therapies to the patients much faster.
By repurposing existing drugs, we make a leap from 5 to 10 years in the development process.
Dr. Ismail
SS: What techniques do you use to develop compounds for rare disease?
Dr. Ismail: We begin by envisaging a suitable target. We use cell culture – either primary cell culture or muscle fiber – to understand the pathophysiology and the consequences of modulating this target. Then, we take a leap from cell culture and identifying the target, into treating mice with selected compounds and evaluate whether the pathology has been alleviated. To do this, we either measure muscle function of the mice in vivo or ex vivo and do a post-mortem analysis to assess many parameters, including histological features.
Prof. Scapozza: We also assess target engagement, which draws on the wide expertise in the group. Firstly, we assess whether the target is linked to the disease and if it is dysregulated, and then we prove that the therapeutic is interacting with our target molecule and that this is restoring the wild type phenotype.
SS: What instruments do you use to carry out your research?
Dr. Ismail: The microplate reader plays a central role in our drug discovery process. We have been the customers of BMG LABTECH for almost 20 years, starting out in 2000 with the FLUOstar® Galaxy* and later the FLUOstar® OPTIMA*, and recently we purchased the CLARIOstar®. BMG LABTECH equipment helps us in many aspects of our research, from analyzing cell culture and biochemical activity, measuring concentration of ions such as calcium and reactive oxygen species in living cells. We also use it to quantify biomarkers of disease in blood samples from treated mice to evaluate the success of the therapy. The readers are versatile and allow us to take fluorescence measurements as well as absorbance and luminescence.
Prof. Scapozza: The other key point we like about the BMG LABTECH microplate readers is their reliability. Our previous instruments have been very reliable and required very little maintenance over the years; we hope that the CLARIOstar will continue this very positive track-record. The instrument is used by over 25 scientists from our group and other groups at the University. It’s becoming the instrument of choice in the department, as it’s reliable and the software is very user friendly.
*Editor’s note: the FLUOstar® Galaxy and OPTIMA are no longer available
I would consider the CLARIOstar crucial equipment in our infrastructure.
Dr. Ismail
SS: Why have you upgraded to the CLARIOstar?
Dr. Ismail: The Galaxy and OPTIMA have been the most stable instruments we have worked with over the years. We upgraded to the CLARIOstar as it contains a monochromator as opposed to filter wheel-based measurement equipment. It gives us a versatility that the previous equipment did not have. The CLARIOstar is much faster, is very precise, can handle many samples in a short time, no matter which application you need to do. I would consider it crucial equipment in our infrastructure.
SS: What are the benefits of the monochromator?
Prof. Scapozza: The key benefit of the monochromator is that it increases the flexibility and versatility of the instrument, allowing it to be utilized in not only the Duchenne muscular dystrophy projects, but all the other projects going on in the lab. As you are not limited to one wavelength, you can better design your experiment by choosing the best wavelength for your molecules excitation.
SS: What are the future goals of the group?
Prof. Scapozza: We have just submitted a paper for peer review about our new finding on Spinal Muscular Atrophy (SMA), where we demonstrated that we could target directly the mRNA of SMN2 to modify splicing, reversing SMA molecular phenotype. This opens a potential therapeutic avenue for other diseases which are linked to mis-splicing or incomplete splicing.
Dr. Ismail: We also have projects on-going and about to go out for submission for treatment of muscular dystrophy and other myopathies. Aside from this we are investigating cell therapies for rare muscle diseases. Our aim will continue to be focused around the swift delivery of therapeutics to a population of patients in high need for such therapies.
Find out more about the CLARIOstar® and other BMG LABTECH instruments.
Do you use BMG LABTECH instruments in your research? Write a review today for your chance to win an Amazon voucher worth $400 or an iPad.

