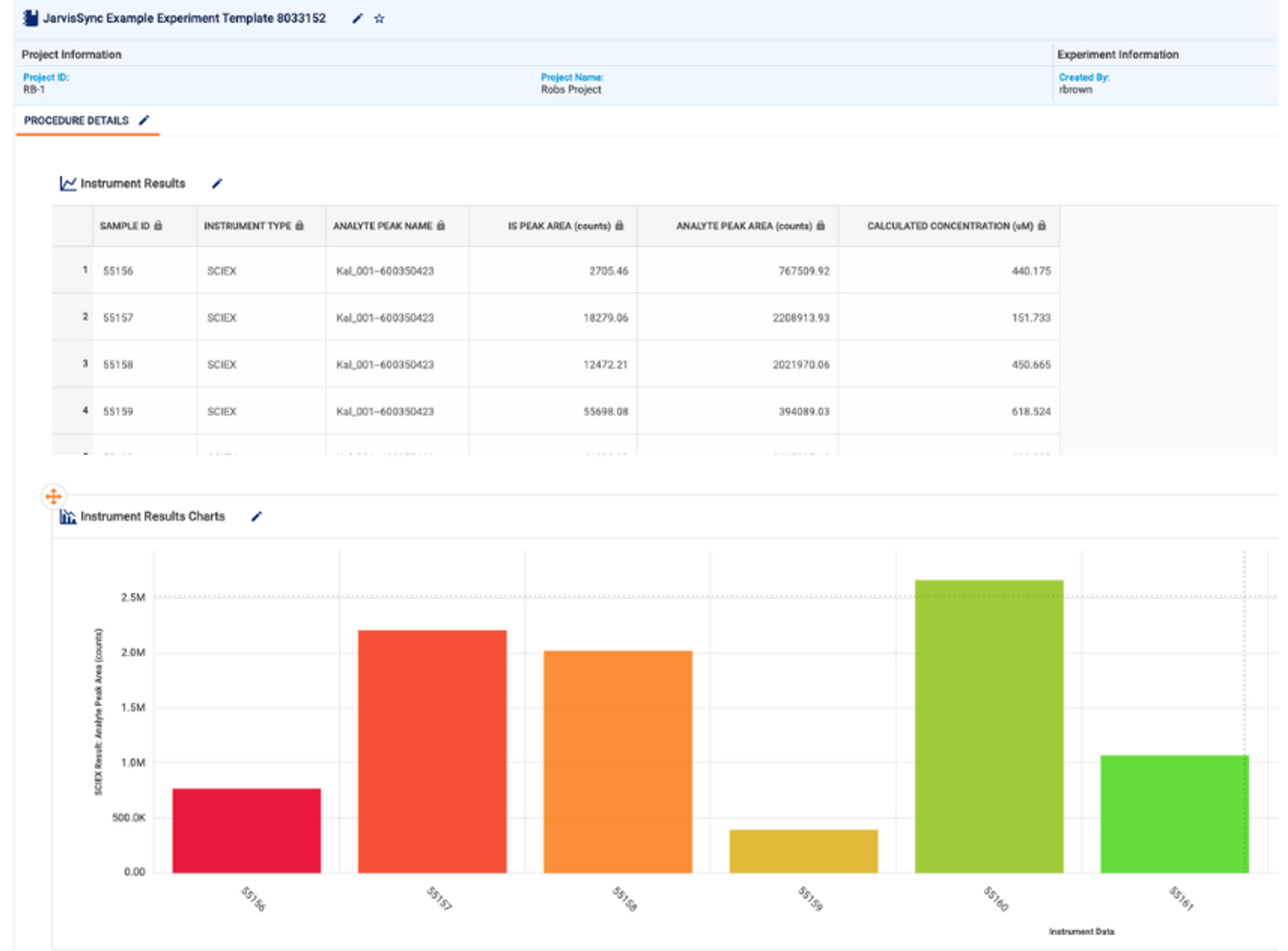
Rethinking lab informatics for the next generation of therapeutics
Biopharma needs informatics platforms that are unified, flexible, and intelligent – ready not just for today’s science, but for what is coming next
13 Aug 2025
Today’s drug discovery pipelines are more diverse than ever. From cell and gene therapies to bispecific antibodies and engineered RNAs, therapeutic innovation is advancing across multiple fronts, and fast. But as therapeutic possibilities expand, and R&D teams increasingly look to integrate artificial intelligence (AI) into their workflows, many labs are discovering their existing informatics infrastructure cannot keep up.

Dr. Rob Brown, Head of the Science Office at Sapio Sciences
Biopharmaceutical companies now face a dual challenge, supporting an increasingly complex scientific landscape, while adopting digital infrastructure capable of evolving with it. Dr. Rob Brown, Head of the Science Office at Sapio Sciences believes addressing this challenge will be critical to the industry’s next wave of discovery.
Fixing the fragmented lab
The biopharma industry is marked by a wide spectrum of digital maturity. Some laboratories are just at the beginning of digitalizing, with workflows heavily reliant on paper, Excel spreadsheets, and file shares. Others have made significant investments in digital tools, deploying Electronic Laboratory Notebooks (ELNs), Laboratory Information Management Systems (LIMS), and Scientific Data Management Systems (SDMS). But even among the more digitally mature, years of layering specialized ELNs, LIMS, and SDMSs have created a patchwork of tools that do not effectively integrate and talk to each other.
“If you adopted a chemistry ELN 10 or 15 years ago, then added a separate one for biologics, and then bolted on a couple of LIMS systems, you may have done the digitization, but you haven’t gone through a transformation,” says Brown. “You can capture data, but it’s not connected in a way that lets you truly exploit it.”
For this reason, researchers are increasingly calling for what Brown refers to as the ‘Holy Grail’ – a single informatics environment capable of unifying data, processes, and context across the entire R&D lifecycle. “A unified system doesn’t just make things easier for IT or finance,” he says. “There are huge scientific advantages when all disciplines involved in a biopharma R&D project can collaborate seamlessly across the entire lifecycle.”
With a unified system, researchers can trace relationships across experiments in different departments and build institutional knowledge that compounds over time. This becomes especially valuable in the context of iterative experimental design. “Without access to data across multiple experiments and departments, decisions under time pressure can be made without full insight,” says Brown. “In R&D, you have to be pragmatic – get what data you can and move on. But if a decision isn’t as fully informed as it could have been, and you go down the wrong research path for a couple of months, the cost is huge.”
If a decision isn’t as fully informed as it could have been, and you go down the wrong research path for a couple of months, the cost is huge.
Dr. Rob Brown, Head of the Science Office Sapio Sciences
New modalities, new needs
Adding to the challenge of managing data across biopharmaceutical R&D is the dramatic shift in the field over the past two decades. Where drug development pipelines were once dominated by small molecules, today’s landscape encompasses a rapidly expanding array of therapeutic modalities. While these new approaches hold tremendous promise, they also introduce scientific and operational complexities that traditional informatics systems were never built to handle.
“Ironically, the newer modalities tend to be more fragmented, because legacy software was developed around traditional modalities like small molecules and peptides – the types of compounds biopharma has been working with for years,” explains Brown.
This gap is especially clear in antibody discovery, where data frequently moves between disparate systems. “There’s a big opportunity for unified systems here,” he continues. “But any solution can’t just fix today’s issues, because in five years, we’ll be facing new modalities and the same challenges again.”
The key to future-proofing informatics, Brown argues, is modality-neutral platforms. This has been employed in Sapio LIMS, which takes a material-centric approach. “Some materials have chemical structures, others have antibody sequences, and some are linked to cell lines or cell therapies, but fundamentally, they’re all materials,” Brown explains. “By building the system around this concept, we created a flexible foundation that can extend to future modalities that haven’t even been invented yet.”
While some biopharma companies require modality-specific infrastructure, Brown points out that large pharma labs often need systems that treat all modalities equally. “Large pharma companies typically have multiple research teams working across many modalities, so they need a system that’s completely neutral to whichever modality proves most appropriate for a given target,” he explains. “Even better, they want to use their data to ask: ‘Next time we have a target, can we predict which modality is likely to work best without testing them all experimentally?’”
“While successful drug modalities get published, companies hold a wealth of information on what didn’t work that never makes it into the public domain,” Brown adds. “Capturing and mining that hidden data is crucial to make faster, smarter decisions moving forward.”
Leveraging AI in ELNs
Beyond the growing diversity of therapeutic modalities, another major shift transforming biopharma is the accelerating adoption of AI, and Sapio’s platform is no exception. It features an embedded scientific assistant called ELaiN, an AI trained to actively use the system on behalf of the scientist.
With ELaiN, researchers can interact with the LIMS or ELN using simple conversational prompts, typed or spoken, removing the need for code-, mouse-, or menu-driven instructions. “Instead of clicking through 50 or 60 steps to create an experiment or plate layout, a scientist can simply ask the AI to do it,” Brown explains. “They can say, ‘Make me a plate, plate the samples, and send it to the Hamilton,’ and it just happens.” This dramatically lowers the barrier to entry, particularly for teams migrating from legacy systems.
The next step, Brown adds, is enabling AI not just to operate the system, but to truly understand the experiments, suggest next steps, and perform complex scientific reasoning. This agentic model of AI will integrate internal data, public datasets, and scientific algorithms to help researchers design better experiments in silico. “It’s like having the head of medicinal chemistry over your shoulder at all times,” he says. “Except now you also have the head of molecular biology, the head of protein engineering, and screening sciences – all ready to answer your question instantly.”
“It’s not about replacing the scientist or the lab,” he emphasizes. “It’s about doing smarter, more targeted lab work. You cut out the trial-and-error steps by running those experiments virtually first.” The result is a ‘lab-in-a-loop system’; scientists use AI to design the best experiments, those experiments generate better data, and that data feeds back into the AI to guide even better decisions next time.
What’s coming next
Brown has no doubt that AI is set to fundamentally reshape life science research. “You only have to look at what it’s already done in language-based fields – writers, developers, designers,” he says. “It’s completely transformed how they work and what they can produce. The same shift is coming to science.”
Two key advances are driving this change. The first is the rise of agentic AI, including models that can actively perform complex tasks like cheminformatics, bioinformatics, and cell image analysis. The second is AI reasoning. “To answer real scientific questions, an AI has to plan experiments, make decisions, and adapt based on outcomes,” Brown explains. “The newest models – ChatGPT-4o, Gemini 2.5 with deep reasoning, Claude – they’re starting to think at a PhD level.”
Together, these capabilities point toward a future where biopharmaceutical R&D will be far more virtual than it is today. Brown envisions a near-term reality in which 80–90% of experimental work is done in silico, with the physical lab reserved for validation and optimization. That shift could also radically widen the scope of discovery. “Where a team today might explore two or three drug modalities due to time and budget constraints, tomorrow’s teams will explore 15 or more, virtually and simultaneously,” he suggests.
And that scale makes a difference. “In real life, maybe you abandon a path too early,” he says. “But with virtual exploration, you can keep going, and you might discover something world-changing you’d have otherwise missed.”